The SpasT-SCI-T trial protocol: Investigating calpain-mediated sodium channel fragments as biomarkers for traumatic CNS injuries and spasticity prediction
- PMID: 40397864
- PMCID: PMC12094745
- DOI: 10.1371/journal.pone.0319635
The SpasT-SCI-T trial protocol: Investigating calpain-mediated sodium channel fragments as biomarkers for traumatic CNS injuries and spasticity prediction
Abstract
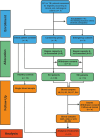
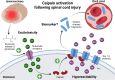
Spinal cord injury and traumatic brain injury are major causes of long-term disability and are often complicated by spasticity, a motor disorder characterized by increased muscle tone and exaggerated reflexes that significantly impair quality of life. Current diagnostic methods lack the sensitivity needed to accurately predict the severity of injury or the onset and progression of spasticity. Trauma-induced calcium dysregulation activates calpains, a family of proteases that cleave sodium channels, disrupting their inactivation and increasing persistent sodium currents. This cascade drives the overexcitability of motoneurons, contributing to the development of spasticity. Consequently, sodium channel fragments have emerged as promising biomarkers that link injury mechanisms to clinical outcomes. The present SpasT-SCI-T clinical trial protocol aims to evaluate sodium channel fragments as blood biomarkers for assessing the severity of spinal cord and traumatic brain injuries, as well as their potential to predict clinical outcomes, including the development of spasticity. This prospective, multicenter, case-control and cohort study involves 40 participants: 20 individuals with spinal cord injury, 10 individuals with traumatic brain injury, and 10 healthy controls. Blood samples are collected within six hours of injury and at follow-up points over six months. Clinical outcomes, including spasticity (assessed using the Modified Ashworth Scale), neurological recovery (measured by the American Spinal Injury Association Impairment Scale and Glasgow Coma Scale), and quality of life (evaluated using the Short Form-36 Health Survey), are analyzed in correlation with biomarker levels. We anticipate that calpain-mediated sodium channel fragments will transform the management of central nervous system injuries by enabling early diagnosis, improving prognostic accuracy, and guiding personalized therapeutic strategies. The clinical trial is registered on ClinicalTrials.gov (NCT06532760, January 10, 2024), with Assistance Publique-Hôpitaux de Marseille as the sponsor.
Copyright: © 2025 Baucher et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Riluzole for treating spasticity in patients with chronic traumatic spinal cord injury: Study protocol in the phase ib/iib adaptive multicenter randomized controlled RILUSCI trial.PLoS One. 2023 Jan 20;18(1):e0276892. doi: 10.1371/journal.pone.0276892. eCollection 2023. PLoS One. 2023. PMID: 36662869 Free PMC article.
-
Predicting the Progression of Spasticity in the Early Phase of Spinal Cord Injury: A Prospective Cohort Study.J Neurotrauma. 2024 May;41(9-10):1122-1132. doi: 10.1089/neu.2023.0191. Epub 2023 Nov 22. J Neurotrauma. 2024. PMID: 37772699
-
Early predictors of developing problematic spasticity following traumatic spinal cord injury: A prospective cohort study.J Spinal Cord Med. 2020 May;43(3):315-330. doi: 10.1080/10790268.2018.1527082. Epub 2018 Oct 9. J Spinal Cord Med. 2020. PMID: 30299227 Free PMC article.
-
[Calpain as a new therapeutic target for treating spasticity after a spinal cord injury].Med Sci (Paris). 2017 Jun-Jul;33(6-7):629-636. doi: 10.1051/medsci/20173306020. Epub 2017 Jul 19. Med Sci (Paris). 2017. PMID: 28990565 Review. French.
-
Effectiveness of 4-Aminopyridine for the Management of Spasticity in Spinal Cord Injury: A Systematic Review.Top Spinal Cord Inj Rehabil. 2018 Fall;24(4):353-362. doi: 10.1310/sci17-00048. Epub 2018 May 3. Top Spinal Cord Inj Rehabil. 2018. PMID: 30459498 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

