Toward a Domain-Overarching Metadata Schema for Making Health Research Studies FAIR (Findable, Accessible, Interoperable, and Reusable): Development of the NFDI4Health Metadata Schema
- PMID: 40397930
- PMCID: PMC12138291
- DOI: 10.2196/63906
Toward a Domain-Overarching Metadata Schema for Making Health Research Studies FAIR (Findable, Accessible, Interoperable, and Reusable): Development of the NFDI4Health Metadata Schema
Erratum in
-
Correction: Toward a Domain-Overarching Metadata Schema for Making Health Research Studies FAIR (Findable, Accessible, Interoperable, and Reusable): Development of the NFDI4Health Metadata Schema.JMIR Med Inform. 2025 Jun 4;13:e78151. doi: 10.2196/78151. JMIR Med Inform. 2025. PMID: 40466103 Free PMC article.
Abstract
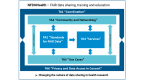
Background: Despite wide acceptance in medical research, implementation of the FAIR (findability, accessibility, interoperability, and reusability) principles in certain health domains and interoperability across data sources remain a challenge. While clinical trial registries collect metadata about clinical studies, numerous epidemiological and public health studies remain unregistered or lack detailed information about relevant study documents. Making valuable data from these studies available to the research community could improve our understanding of various diseases and their risk factors. The National Research Data Infrastructure for Personal Health Data (NFDI4Health) seeks to optimize data sharing among the clinical, epidemiological, and public health research communities while preserving privacy and ethical regulations.
Objective: We aimed to develop a tailored metadata schema (MDS) to support the standardized publication of health studies' metadata in NFDI4Health services and beyond. This study describes the development, structure, and implementation of this MDS designed to improve the FAIRness of metadata from clinical, epidemiological, and public health research while maintaining compatibility with metadata models of other resources to ease interoperability.
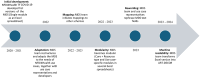
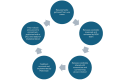
Methods: Based on the models of DataCite, ClinicalTrials.gov, and other data models and international standards, the first MDS version was developed by the NFDI4Health Task Force COVID-19. It was later extended in a modular fashion, combining generic and NFDI4Health use case-specific metadata items relevant to domains of nutritional epidemiology, chronic diseases, and record linkage. Mappings to schemas of clinical trial registries and international and local initiatives were performed to enable interfacing with external resources. The MDS is represented in Microsoft Excel spreadsheets. A transformation into an improved and interactive machine-readable format was completed using the ART-DECOR (Advanced Requirement Tooling-Data Elements, Codes, OIDs, and Rules) tool to facilitate editing, maintenance, and versioning.
Results: The MDS is implemented in NFDI4Health services (eg, the German Central Health Study Hub and the Local Data Hub) to structure and exchange study-related metadata. Its current version (3.3) comprises 220 metadata items in 5 modules. The core and design modules cover generic metadata, including bibliographic information, study design details, and data access information. Domain-specific metadata are included in use case-specific modules, currently comprising nutritional epidemiology, chronic diseases, and record linkage. All modules incorporate mandatory, optional, and conditional items. Mappings to the schemas of clinical trial registries and other resources enable integrating their study metadata in the NFDI4Health services. The current MDS version is available in both Excel and ART-DECOR formats.
Conclusions: With its implementation in the German Central Health Study Hub and the Local Data Hub, the MDS improves the FAIRness of data from clinical, epidemiological, and public health research. Due to its generic nature and interoperability through mappings to other schemas, it is transferable to services from adjacent domains, making it useful for a broader user community.
Keywords: FAIR; accessibility; chronic diseases; clinical trial; data models; data sharing; epidemiological; epidemiology; findability; health research; implementation; interoperability development; metadata standards; nutrition; public health; reusability; structure; tool.
©Haitham Abaza, Aliaksandra Shutsko, Sophie A I Klopfenstein, Carina N Vorisek, Carsten Oliver Schmidt, Claudia Brünings-Kuppe, Vera Clemens, Johannes Darms, Sabine Hanß, Timm Intemann, Franziska Jannasch, Elisa Kasbohm, Birte Lindstädt, Matthias Löbe, Katharina Nimptsch, Ute Nöthlings, Marisabel Gonzalez Ocanto, Tracy Bonsu Osei, Ines Perrar, Manuela Peters, Tobias Pischon, Ulrich Sax, Matthias B Schulze, Florian Schwarz, Carolina Schwedhelm, Sylvia Thun, Dagmar Waltemath, Atinkut A Zeleke, Wolfgang Müller, Martin Golebiewski. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 21.05.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Schwedhelm C, Nimptsch K, Ahrens W, Hasselhorn HM, Jöckel KH, Katzke V, Kluttig A, Linkohr B, Mikolajczyk R, Nöthlings Ute, Perrar I, Peters A, Schmidt CO, Schmidt B, Schulze MB, Stang A, Zeeb H, Pischon T. Chronic disease outcome metadata from German observational studies - public availability and FAIR principles. Sci Data. 2023;10(1):868. doi: 10.1038/s41597-023-02726-7. https://doi.org/10.1038/s41597-023-02726-7 10.1038/s41597-023-02726-7 - DOI - PMC - PubMed
-
- Protocol Registration Data Element Definitions for Interventional and Observational Studies. U.S. National Library of Medicine. ClinicalTrials.gov. 2020. [2024-02-04]. https://clinicaltrials.gov/policy/protocol-definitions .
-
- Federal Institute for Drugs and Medical Devices German Clinical Trials Register. BfArM. 2020. [2024-02-04]. https://www.bfarm.de/EN/BfArM/Tasks/German-Clinical-Trials-Register/_nod... .
-
- World Health Organization. International Clinical Trials Registry Platform (ICTRP) 2020. [2024-02-06]. https://www.who.int/clinical-trials-registry-platform/
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

