Modelling adrenal steroid profiles to inform monitoring guidance in congenital adrenal hyperplasia
- PMID: 40398353
- PMCID: PMC12148605
- DOI: 10.1016/j.ebiom.2025.105749
Modelling adrenal steroid profiles to inform monitoring guidance in congenital adrenal hyperplasia
Abstract
Background: There is no consensus on how to monitor adrenal androgens in Congenital Adrenal Hyperplasia (CAH).
Methods: Modelling of serum and salivary steroid profiles in healthy participants and patients with CAH randomised to either standard treatment or modified-release hydrocortisone hard capsules (MRHC).
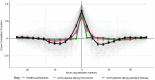
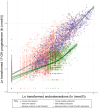
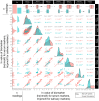
Findings: Changes in serum 17-hydroxyprogesterone (17OHP) and androstenedione (A4) paralleled each other in healthy participants (n = 19) and patients with CAH (n = 122). However, healthy participants had similar absolute levels of 17OHP and A4 whereas patients with CAH had proportionally higher levels of 17OHP. Cross-correlation showed no lag between serum 17OHP and A4. In CAH, Bayesian multiple change point analysis converged on a 17OHP of 4.5 nmol/l below which in proportion to 17OHP the A4 is lower. Patients on standard treatment had a morning peak in 17OHP and A4 whereas patients on MRHC had relatively flat profiles. Salivary androgens including 11-ketotestosterone correlated with serum 17OHP and A4 in female patients (r = 0.7 to 0.9).
Interpretation: In CAH, elevated 17OHP drives the production of A4. High A4 reflects poor control, but low A4 does not indicate overtreatment. Accepting 17OHP is higher than A4, both measurements give similar reflection of control, and a 17OHP <38 nmol/l (1250 ng/dl) was associated with an A4 in the normal range <5 nmol/l (143 ng/dl) in 95% of patients and in clinical trials was used to define good control. On MRHC, which controls androgen levels over 24 h, a single sample of 17OHP and/or A4 can be used to monitor control. Salivary measurements reflect similar results to serum.
Funding: Diurnal; MRC; NIH; NIHR.
Keywords: 21-Hydroxylase deficiency; Adrenal insufficiency; Congenital adrenal hyperplasia; Glucocorticoid; Hydrocortisone; Modified release hydrocortisone; Monitoring.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests A.P., E.B., D.P.M., J.N-P., A.R., N.K. and R.R. report additional COIs: R.R. is a Director of Diurnal Group Plc. A.P. receives unrelated research funds from Diurnal Limited and HRA Pharma. D.A.R. has received honoraria from Neurocrine biosciences. N.R. has received honoraria from Lundbeck, Crinetics, Spruce Biosciences and Neurocrine Biosciences. J.N-P. has received unrelated research funds from Diurnal Group Plc and Crinetics. D.M. received unrelated research funds from Diurnal Limited, Neurocrine Biosciences and Adrenas Therapeutics, all through the National Institutes of Health Cooperative Research and Development Agreements. N.K. has received research funds from Neurocrine Biosciences. G.C. is a NIHR Senior Investigator. A.P. delivered results for this study through the NIHR Birmingham Biomedical Research Centre. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care.
Figures

References
-
- Pang S.Y., Wallace M.A., Hofman L., et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988;81(6):866–874. - PubMed
-
- White P.C., Speiser P.W. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21(3):245–291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

