Age-Related Oxidative Stress and Mitochondrial Dysfunction in Lymph Node Stromal Cells Limit the Peripheral T Cell Homeostatic Maintenance and Function
- PMID: 40398422
- PMCID: PMC12341786
- DOI: 10.1111/acel.70100
Age-Related Oxidative Stress and Mitochondrial Dysfunction in Lymph Node Stromal Cells Limit the Peripheral T Cell Homeostatic Maintenance and Function
Abstract
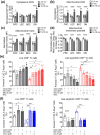
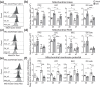
Lymph nodes (LN) are the key organs in charge of long-term maintenance of naïve lymphocytes and their initial, primary activation upon infection. Accumulating evidence indicates that LN stromal cells undergo degenerative changes with aging that critically impair LN function, including the generation of protective primary immune responses. The nature of these defects remains incompletely understood. We here demonstrate that age-related LN stromal changes manifest themselves in mitochondrial dysfunction and oxidative stress. Ex vivo, all three major stromal cell subsets, fibroblastic reticular cells (FRC), lymphatic endothelial cells (LEC), and blood endothelial cells (BEC) exhibit elevated mitochondrial reactive oxygen species (ROS) stress, reduced mitochondrial potential, and elevated mitochondrial mass with aging. Old FRC also exhibited elevated cytoplasmic ROS production. This was accompanied by the reduced ability of old LN stromal cells to support Tn survival in vitro, a defect alleviated by pretreating old LN stroma with the general antioxidant N-acetyl cysteine (NAC) as well as by mitochondrial ROS-reducing (mitoquinone) and mitophagy-inducing (urolithin A) compounds. Mitochondrial dysfunction and, in particular, reduced mitochondrial potential in old FRC were also seen upon vaccination or infection in vivo. Consistent with these results, in vivo antioxidant treatment of old mice with NAC restored to adult levels the numbers of antigen-specific CD8+ effector T cells and their production of granzyme B in response to antigenic challenge.
Keywords: T cell homeostasis; aging; lymph node stromal cells; mitochondrial dysfunction; oxidative stress.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

