Decoding Lusichelins A-E: An In-Depth Look at the Metallophores of Lusitaniella coriacea LEGE 07167-Structure, Production, and Functionality
- PMID: 40398867
- PMCID: PMC12210259
- DOI: 10.1021/acs.jnatprod.5c00204
Decoding Lusichelins A-E: An In-Depth Look at the Metallophores of Lusitaniella coriacea LEGE 07167-Structure, Production, and Functionality
Abstract
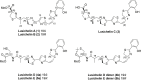
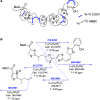
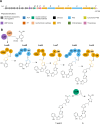
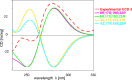
Essential trace metals are vital for cellular processes, such as respiration, DNA replication, and photosynthesis. Cyanobacteria must tightly regulate metal homeostasis to prevent deficiency or toxicity, yet their metallophores remain overlooked. Here, we report lusichelins A-E (1-5), new metallophores isolated from the marine cyanobacterium Lusitaniella coriacea LEGE 07167. Their structures and configurational assignments were determined by using NMR, mass spectrometry, TD-DFT calculations, and retrobiosynthetic insights. Lusichelins feature a unique structural arrangement with thiazoline/thiazole rings connected via a vinyl group, an aliphatic carbon chain, or directly enabling the potential for metal coordination. Genomic analysis identified a hybrid PKS/NRPS biosynthetic gene cluster consistent with the lusichelin structure, bearing traits characteristic of metallophore biosynthesis. Functionally, lusichelins act as metallophores capable of chelating both iron and copper. Lusichelin C (3) consistently bound iron under both metal-rich and metal-limited culture conditions, while copper complexation was only observed under elevated copper levels. At physiologically relevant pH values, no significant metal-binding preference was detected. Moreover, compound production was maximized under metal-rich conditions and in response to copper limitation. Lusichelin B (2) exhibited cytotoxicity against colon carcinoma cells while reversing multidrug resistance via ABCB1 efflux pump modulation. These findings expand our understanding of cyanobacterial metallophores in microbial metal homeostasis and highlight their biological potential.
Figures


Similar articles
-
Leptochelins A-C, Cytotoxic Metallophores Produced by Geographically Dispersed Leptothoe Strains of Marine Cyanobacteria.J Am Chem Soc. 2024 Jul 10;146(27):18626-18638. doi: 10.1021/jacs.4c05399. Epub 2024 Jun 25. J Am Chem Soc. 2024. PMID: 38918178 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- El-Seedi H. R., El-Mallah M. F., Yosri N., Alajlani M., Zhao C., Mehmood M. A., Du M., Ullah H., Daglia M., Guo Z.. et al. Review of Marine Cyanobacteria and the Aspects Related to Their Roles: Chemical, Biological Properties, Nitrogen Fixation and Climate Change. Mar. Drugs. 2023;21(8):439. doi: 10.3390/md21080439. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

