2-(Cyanomethyl)benzimidazole Derivatives as 1,3-Dicarbonyl Analogues for a Kinetically Controlled Diastereo- and Enantioselective Mannich-Type Reaction Catalyzed by Chiral Phosphoric Acid
- PMID: 40398870
- PMCID: PMC12150310
- DOI: 10.1021/acs.orglett.5c01478
2-(Cyanomethyl)benzimidazole Derivatives as 1,3-Dicarbonyl Analogues for a Kinetically Controlled Diastereo- and Enantioselective Mannich-Type Reaction Catalyzed by Chiral Phosphoric Acid
Abstract
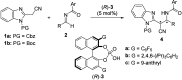
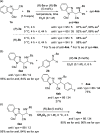
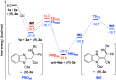
An asymmetric Mannich-type reaction of N-protected 2-(cyanomethyl)benzimidazoles with N-benzoyl imines was developed by using chiral phosphoric acid as a chiral Brønsted acid catalyst. Products having vicinal trisubstituted carbon stereogenic centers were formed in a highly diastereo- and enantioselective manner, even though one of the stereogenic centers had an active methine proton. Comprehensive control experiments revealed that high stereoselectivity was achieved through a kinetically controlled process.
Figures
Similar articles
-
A Powerful Chiral Phosphoric Acid Catalyst for Enantioselective Mukaiyama-Mannich Reactions.Angew Chem Int Ed Engl. 2016 Jul 25;55(31):8970-4. doi: 10.1002/anie.201603929. Epub 2016 Jun 6. Angew Chem Int Ed Engl. 2016. PMID: 27265881
-
Chiral brønsted Acid-catalyzed stereoselective Mannich-type reaction of azlactones with aldimines.J Org Chem. 2015 Jan 2;80(1):590-4. doi: 10.1021/jo5024975. Epub 2014 Dec 12. J Org Chem. 2015. PMID: 25469764
-
Construction of Vicinal Quaternary Stereogenic Centers by Enantioselective Direct Mannich-Type Reaction Using a Chiral Bis(guanidino)iminophosphorane Catalyst.Angew Chem Int Ed Engl. 2016 Apr 4;55(15):4734-7. doi: 10.1002/anie.201601352. Epub 2016 Mar 8. Angew Chem Int Ed Engl. 2016. PMID: 26954063
-
Chiral Phosphoric Acid Catalyzed Diastereo- and Enantioselective Mannich-Type Reaction between Enamides and Thiazolones.Org Lett. 2016 Jun 3;18(11):2521-3. doi: 10.1021/acs.orglett.6b00857. Epub 2016 May 13. Org Lett. 2016. PMID: 27175571
-
Catalytic asymmetric Mannich reactions of glycine derivatives with imines. A new approach to optically active alpha,beta-diamino acid derivatives.J Org Chem. 2003 Apr 4;68(7):2583-91. doi: 10.1021/jo026766u. J Org Chem. 2003. PMID: 12662026
References
-
-
For reviews of benzimidazole derivatives as pharmerceutical chromophores, see:
- Rajasekhar S., Maiti B., Balamurali M. M., Chanda K.. Synthesis and Medicinal Applications of Benzimidazoles: An Overview. Curr. Org. Synth. 2016;14:40–60. doi: 10.2174/1570179413666160818151932. - DOI
- Patel A., Shah D., Patel N., Patel K., Soni N., Nagani A., Parikh V., Shah H., Bambharoliya T.. Benzimidazole as Ubiquitous Structural Fragment: An Update on Development of its Green Synthetic Approaches. Mini-Rev. Org. Chem. 2021;18:1064–1085. doi: 10.2174/1570193X17999201211194908. - DOI
- Monga J., Ghosh N. S., Rani I., Singh R., Deswal G., Dhingra A. K., Grewal A. S.. Unlocking the Pharmacological Potential of Benzimidazole Derivatives: A Pathway to Drug Development. Curr. Top. Med. Chem. 2024;24:437–485. doi: 10.2174/0115680266283641240109080047. - DOI - PubMed
- El Alami A., Sdassi H., Bouzikri S.. Review of Synthesis Process of Benzimidazole-heterocycle Hybrid Compounds. Synth. Commun. 2024;54:613–635. doi: 10.1080/00397911.2024.2316718. - DOI
-
-
-
For Brønsted acid-promoted functionalization at the α-position of azaarene derivatives, see:
- Niu R., Xiao J., Liang T., Li X.. Facile Synthesis of Azaarene-Substituted 3-Hydroxy-2-Oxindoles via Brønsted Acid Catalyzed sp3 C–H Functionalization. Org. Lett. 2012;14:676–679. doi: 10.1021/ol2030982. - DOI - PubMed
- Wang F. F., Luo C. P., Wang Y., Deng G., Yang L.. Brønsted Acid Promoted Benzylic C–H Bond Functionalization of Azaarenes: Nucleophilic Addition to Aldehydes. Org. Biomol. Chem. 2012;10:8605–8608. doi: 10.1039/c2ob26604k. - DOI - PubMed
- Li H. Y., Xing L. J., Xu T., Wang P., Liu R. H., Wang B.. An Addition of Benzylic sp3 C–H to Electron-Deficient Olefins. Tetrahedron Lett. 2013;54:858–860. doi: 10.1016/j.tetlet.2012.11.100. - DOI
- Jin J. J., Wang D. C., Niu H. Y., Wu S., Qu G. R., Zhang Z. B., Guo H. M.. Brønsted Acid Catalyzed Synthesis of 1,3-Di(2-Quinolyl)Propane Derivatives via Tandem C(sp3)–H Functionalization. Tetrahedron. 2013;69:6579–6584. doi: 10.1016/j.tet.2013.05.135. - DOI
- Lansakara A. I., Farrell D. P., Pigge F. C.. Brønsted Acid Catalyzed Intramolecular Benzylic Cyclizations of Alkylpyridines. Org. Biomol. Chem. 2014;12:1090–1099. doi: 10.1039/C3OB42039F. - DOI - PubMed
- Wang F. F., Luo C. P., Deng G., Yang L.. C(sp3)–C(sp3) Bond Formation via Copper/Brønsted Acid Co-Catalyzed C(sp3)–H Bond Oxidative Cross-Dehydrogenative-Coupling (CDC) of Azaarenes. Green Chem. 2014;16:2428–2431. doi: 10.1039/c4gc00038b. - DOI
- Gao X., Zhang F., Deng G., Yang L.. Brønsted Acid Catalyzed Benzylic C–H Bond Functionalization of Azaarenes: Nucleophilic Addition to Nitroso Compounds. Org. Lett. 2014;16:3664–3667. doi: 10.1021/ol501422k. - DOI - PubMed
- Zhu Z. Q., Bai P., Huang Z. Z.. Dehydrogenative Cross-Coupling Reaction by Cooperative Transition-Metal and Brønsted Acid Catalysis for the Synthesis of β-Quinolinyl α-Amino Acid Esters. Org. Lett. 2014;16:4881–4883. doi: 10.1021/ol502402s. - DOI - PubMed
-
-
-
For reviews on enantioselective reactions of azaarene derivatives, see:
- Best D., Lam H. W.. C = N-Containing Azaarenes as Activating Groups in Enantioselective Catalysis. J. Org. Chem. 2014;79:831–845. doi: 10.1021/jo402414k. - DOI - PubMed
- Meazza M., Rios R.. Enantioselective Synthesis of Alkyl Azaarenes. Asian J. Org. Chem. 2019;8:1800–1812. doi: 10.1002/ajoc.201900363. - DOI
-
-
-
For 2-alkylazaarene derivatives as the nucleophile for enantioselective reactions, see:
- Trost B. M., Thaisrivongs D. A., Hartwig J.. Palladium-Catalyzed Asymmetric Allylic Alkylations of Polynitrogen-Containing Aromatic Heterocycles. J. Am. Chem. Soc. 2011;133:12439–12441. doi: 10.1021/ja205523e. - DOI - PubMed
- Vera S., Liu Y., Marigo M., Escudero-Adán E. C., Melchiorre P.. Asymmetric Michael Addition of Nitrobenzyl Pyridines to Enals via Iminium Catalysis. Synlett. 2011;2011:489–494. doi: 10.1055/s-0030-1259518. - DOI
- Fallan C., Lam H. W.. Enantioselective Nickel-Catalyzed Michael Additions of Azaarylacetates and Acetamides to Nitroalkenes. Chem. - Eur. J. 2012;18:11214–11218. doi: 10.1002/chem.201202093. - DOI - PubMed
- Best D., Kujawa S., Lam H. W.. Diastereo- and Enantioselective Pd(II)-Catalyzed Additions of 2-Alkylazaarenes to N-Boc Imines and Nitroalkenes. J. Am. Chem. Soc. 2012;134:18193–18196. doi: 10.1021/ja3083494. - DOI - PubMed
- Bastida I., San Segundo M., Lopez R., Palomo C.. Strategy for Stereoselective Metal-free α-Functionalization of 2-Azaaryl Acetates with N-Boc Imines. Chem. - Eur. J. 2017;23:13332–13336. doi: 10.1002/chem.201703748. - DOI - PubMed
- Li J., Fu Y., Qin C., Yu Y., Li H., Wang W.. Asymmetric Synthesis of Isoquinolinonaphthyridines Catalyzed by a Chiral Brønsted Acid. Org. Biomol. Chem. 2017;15:6474–6477. doi: 10.1039/C7OB01527E. - DOI - PubMed
- Jiang X., Boehm P., Hartwig J. F.. Stereodivergent Allylation of Azaaryl Acetamides and Acetates by Synergistic Iridium and Copper Catalysis. J. Am. Chem. Soc. 2018;140:1239–1242. doi: 10.1021/jacs.7b12824. - DOI - PMC - PubMed
- Hu Q., Kondoh A., Terada M.. Enantioselective Direct Mannich-Type Reactions of 2-Benzylpyridine N-Oxides Catalyzed by Chiral Bis(guanidino)iminophosphorane Organosuperbase. Chem. Sci. 2018;9:4348–4351. doi: 10.1039/C8SC00808F. - DOI - PMC - PubMed
- Wang Y., Wang K., Cao W., Liu X., Feng X.. Diastereo- and Enantioselective 1,6-Conjugate Addition of 2-Azaarylacetamides to Para-Quinone Methides. Org. Lett. 2019;21:6063–6067. doi: 10.1021/acs.orglett.9b02215. - DOI - PubMed
- Meazza M., Rios R.. Highly Regio- and Enantioselective Organocatalytic γ-Allylic Alkylation of Quinolines. Adv. Synth. Catal. 2021;363:1341–1345. doi: 10.1002/adsc.202001213. - DOI
- Shen Y.-B., Qian H.-L., Yang L., Zhou S., Rao H.-W., Wang Z.-H., You Y., Zhang Y.-P., Yin J.-Q., Zhao J.-Q., Zhang W., Yuan W.-C.. Cu-Catalyzed Direct Asymmetric Mannich Reaction of 2-Alkylazaarenes and Isatin-Derived Ketimines. Org. Lett. 2024;26:1699–1704. doi: 10.1021/acs.orglett.4c00227. - DOI - PubMed
-
-
-
For enantioselective construction of tetrasubstituted carbon using azaarene derivatives as nucleophiles, see:
- Izquierdo J., Landa A., Bastida I., López R., Oiarbide M., Palomo C.. Base-Catalyzed Asymmetric α-Functionalization of 2-(Cyanomethyl)azaarene N-Oxides Leading to Quaternary Stereocenters. J. Am. Chem. Soc. 2016;138:3282–3285. doi: 10.1021/jacs.5b13385. - DOI - PubMed
- Meazza M., Potter M., Pitak M. B., Coles S. J., Mazzanti A., Rios R.. Highly Enantioselective Synthesis of Alkylpyridine Derivatives through a Michael/Michael/Aldol Cascade Reaction. Eur. J. Org. Chem. 2017;2017:719–725. doi: 10.1002/ejoc.201601491. - DOI
- Wang K., Chen C., Liu X., Li D., Peng T., Liu X., Yang D., Wang L.. Enantioselective Reaction between 2-(Cyanomethyl)Azaarenes and N-Boc-Amino Sulfones. Org. Lett. 2018;20:5260–5263. doi: 10.1021/acs.orglett.8b02205. - DOI - PubMed
- Das A., Joshi H., Singh V. K.. Asymmetric α-Functionalization of 2-Alkyl Azaarenes: Synthesis of Tertiary Fluorides Having Vicinal Stereogenic Centers. Org. Lett. 2021;23:9441–9445. doi: 10.1021/acs.orglett.1c03626. - DOI - PubMed
- Wang Z.-Q., Liu Z.-C., Huang X., Yin L.. Construction of Halogenated Tetrasubstituted Carbon Centers Through Copper(I)-catalyzed Asymmetric Alkylation of 2-Azaarylesters. Cell Rep. Phys. Sci. 2024;5:101822. doi: 10.1016/j.xcrp.2024.101822. - DOI
- Wen H.-C., Chen W., Li M., Ma C., Wang J.-F., Fu A., Xu S.-Q., Zhou Y.-F., Ni S.-F., Mao B.. Chiral Phosphoric Acid-catalyzed Asymmetric Epoxidation of Alkenyl Aza-heteroarenes Using Hydrogen Peroxide. Nat. Commun. 2024;15:5277. doi: 10.1038/s41467-024-49435-2. - DOI - PMC - PubMed
- Lapray A., Hiebel M.-A., Oudeyer S., Lohier J.-F., Suzenet F., Brière J.-F.. 3-Alkyl-1,2,4-triazines as Heterocyclic Platforms for Organocatalytic Enantioselective Benzylic C–H Functionalization. Org. Lett. 2025;27:1504–1510. doi: 10.1021/acs.orglett.5c00008. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

