Mesenchymal intravenous stromal cell infusions in children with recessive dystrophic epidermolysis bullosa: MissionEB protocol for a randomised, double-blinded, placebo-controlled, two-centre, crossover trial with an internal phase I dose de-escalation phase and open-label extension
- PMID: 40398934
- PMCID: PMC12097048
- DOI: 10.1136/bmjopen-2024-089857
Mesenchymal intravenous stromal cell infusions in children with recessive dystrophic epidermolysis bullosa: MissionEB protocol for a randomised, double-blinded, placebo-controlled, two-centre, crossover trial with an internal phase I dose de-escalation phase and open-label extension
Abstract
Introduction: Recessive dystrophic epidermolysis bullosa (RDEB) is a severe genetic mucocutaneous fragility disorder characterised by chronic blistering, slow wound healing and increased risk of squamous cell carcinoma. Current management options are very limited.
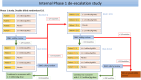
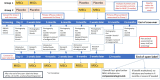
Methods: This is a randomised (1:1), placebo-controlled, double-blinded crossover (A/B) trial with an internal phase I dose de-escalation (4+5 design) in the first 3 months and a 12-month continued treatment follow-on open-label study if 3-month outcome data from the crossover trial indicate safe and beneficial effects. RDEB is a rare condition, so we expect to recruit a maximum of 36 participants based on feasibility and not formal power considerations. Participants aged>6 months and <16 years will be recruited at Great Ormond Street Hospital and Birmingham Children's Hospital. They will receive 2-3×106 cells/kg intravenous infusion of umbilical cord-derived mesenchymal stem cells or placebo at the start of each crossover period (day 0) and 14 days later. The dose will be de-escalated to 1-1.5×106 cells/kg depending on observed toxicity. For the main crossover trial, the primary outcome is the change in disease severity as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index at 3 months from day 0 infusion. Secondary outcomes measured at 3 and 6 months from day 0 infusion include changes in general clinical appearance of skin disease, pain and itch, and quality of life. Adverse events and serious adverse events will be monitored throughout the trial.
Ethics and dissemination: North East-York Research Ethics Committee approved the protocol (ref: 21/NE/0016) on 16 March 2021. Findings will be published in peer-reviewed scientific journals, presented at relevant national and international conferences, and an open-access final report submitted to the funder.
Trial registration number: ISRCTN14409785. Protocol V. 8.0, 14 November 2022.
Keywords: Clinical Trial; DERMATOLOGY; Mesenchymal Stem Cells; Paediatric dermatology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: MLB is contracted as a subinvestigator by Rheacell and Twi Biotechnology Inc. AEM is an investigator for ongoing clinical trials with Rheacell, TWi Biotechnology and served as a consultant for KrystalBio. GP receives payment or honoraria for lectures, presentation, speaker bureaus, manuscript writing or educational events from Krystal Biotech, Amryt Pharma and Incyte Corporation, and support for attending meetings or travel from SANOFI. All other authors have no competing interests to declare.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
