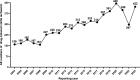
Assessing the association between drug use and ischaemic colitis: a retrospective pharmacovigilance study using FDA Adverse Event data
- PMID: 40398943
- PMCID: PMC12097096
- DOI: 10.1136/bmjopen-2024-088512
Assessing the association between drug use and ischaemic colitis: a retrospective pharmacovigilance study using FDA Adverse Event data
Abstract
Objective: Drug-induced ischaemic colitis is a significant adverse event (AE) in clinical practice. This study aimed to recognise the top drugs associated with the risk of ischaemic colitis based on the FDA Adverse Event Reporting System (FAERS) database.
Design: A cross-sectional design.
Setting: All data retrieved from the FAERS database from the first quarter of 2004 to the fourth quarter of 2023.
Participants: A total of 5664 drug-induced ischaemic colitis AEs eligible for screening.
Primary and secondary outcome measures: The Medical Dictionary for Regulatory Activities was used to identify ischaemic colitis (code: 10009895) cases. Disproportionality analysis for drug-associated ischaemic colitis signals.
Results: Drug-induced ischaemic colitis AEs were more prevalent in females (60.12%) and individuals aged ≥65 years (34.25%). The common outcomes were hospitalisation (46.85%) and death (9.73%). Disproportionality analysis identified 91 ischaemic colitis signals and the top 30 drugs mainly involved in the gastrointestinal and nervous systems. The top five drugs with the highest reported OR, proportional reporting ratio, information component and the empirical Bayesian geometric mean, were alosetron, tegaserod, osmoprep, naratriptan and kayexalate. Additionally, 20 of the top 30 drugs did not have ischaemic colitis risk indicated in the package insert.
Conclusions: This study identified key drugs associated with ischaemic colitis, particularly alosetron, tegaserod, osmoprep, naratriptan and kayexalate. Notably, two-thirds of these drugs lacked ischaemic colitis warnings in their package inserts. These findings underscore the need for greater clinical vigilance, improved regulatory oversight and further research to clarify underlying mechanisms and support safer medication use.
Keywords: Adverse events; Drug Utilization; Gastroduodenal disease.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
The drug risks of cilostazol: A pharmacovigilance study of FDA Adverse Event Reporting System database.PLoS One. 2024 Dec 4;19(12):e0314957. doi: 10.1371/journal.pone.0314957. eCollection 2024. PLoS One. 2024. PMID: 39630707 Free PMC article.
-
A post-marketing pharmacovigilance study of avapritinib: Adverse event data mining and analysis based on the United States Food and Drug Administration Adverse Event Reporting System database.Br J Clin Pharmacol. 2024 Aug;90(8):1816-1826. doi: 10.1111/bcp.15673. Epub 2023 Feb 6. Br J Clin Pharmacol. 2024. PMID: 36702463
-
Drug-induced hypoglycemia: a disproportionality analysis of the FAERS database.Expert Opin Drug Saf. 2024 Aug;23(8):1061-1067. doi: 10.1080/14740338.2023.2278700. Epub 2023 Nov 9. Expert Opin Drug Saf. 2024. PMID: 37909653
-
Romosozumab adverse event profile: a pharmacovigilance analysis based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023.Aging Clin Exp Res. 2025 Jan 14;37(1):23. doi: 10.1007/s40520-024-02921-5. Aging Clin Exp Res. 2025. PMID: 39808360 Free PMC article.
-
Vitiligo associated with type 2 immune inhibitors: FAERS analysis and literature review.J Dermatol. 2025 Jun;52(6):1008-1014. doi: 10.1111/1346-8138.17698. Epub 2025 Mar 15. J Dermatol. 2025. PMID: 40087891 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical