Gene therapy prevents onset of mitochondrial cardiomyopathy in neonatal mice with Ndufs6 deficiency
- PMID: 40399258
- PMCID: PMC12095822
- DOI: 10.1038/s41420-025-02524-7
Gene therapy prevents onset of mitochondrial cardiomyopathy in neonatal mice with Ndufs6 deficiency
Abstract
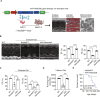
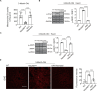
Mutations in genes affecting mitochondrial complex I (CI) can lead to mitochondrial cardiomyopathy (MCM) yet no effective treatment. This study sought to determine whether adeno-associated virus 9 (AAV9)-based gene therapy could prevent or rescue Ndufs6 deficiency-induced MCM at different disease stages. Using Ndufs6gt/gt mice to mimic MCM, cardiac dysfunction was evident at week 4 post-birth, showing reduced ejection fraction, CI activity, increased fibrosis, mitochondrial fission, and disrupted cristae. Neonatal and adult mice were intravenously given AAV9-hNdufs6 (1e14 vg kg-1). AAV9-hNdufs6 therapy effectively prevented neonatal mice's cardiac dysfunction onset, preserving CI activity and cristae structure for 11 months. In contrast, therapy in adult mice post-disease onset failed to reverse or halt progression of heart dilation and failure after 3 months, showing mitochondrial abnormalities and cardiomyocyte apoptosis. Mechanistically, adult mouse Kupffer cells demonstrated enhanced phagocytic capabilities compared to neonatal mice, with higher expression levels of AAV9 cell surface receptors observed in neonatal mouse hearts, rendering neonatal mice more responsive to AAV9-mediated gene therapy for heart tissue. Additionally, AAV9-hNdufs6 gene therapy initiated at an early stage increased Ndufs6 expression in cardiac tissue, preserved mitochondrial structure and function, prevented cardiomyocyte fibrosis through modulation of the AMPK/Drp1 signaling pathway. In conclusion, early intervention with AAV9-hNdufs6 gene therapy can effectively prevent the onset of MCM, but intervention after disease onset has limited efficacy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing of interests.
Figures





References
-
- Kang L, Jin S, Wang J, Lv Z, Xin C, Tan C, et al. AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. J Control Release. 2023;355:458–73. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

