Exercise-induced CLCF1 attenuates age-related muscle and bone decline in mice
- PMID: 40399268
- PMCID: PMC12095553
- DOI: 10.1038/s41467-025-59959-w
Exercise-induced CLCF1 attenuates age-related muscle and bone decline in mice
Abstract
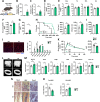
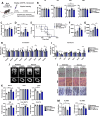
Skeletal muscle undergoes many alterations with aging. However, the impact of aging on muscle's ability to secrete myokines and its subsequent effects on the body remain largely unexplored. Here, we identify myokines that have the potential to ameliorate age-related muscle and bone decline. Notably, circulating levels of cardiotrophin-like cytokine factor 1 (CLCF1) decrease with age, while exercise significantly upregulates CLCF1 levels in both humans and rodents. Restoring CLCF1 levels in aged male mice improves their physical performance, glucose tolerance, and mitochondrial activity. Furthermore, CLCF1 protects against age-induced bone loss by inhibiting osteoclastogenesis and promoting osteoblast differentiation in aged male mice. These improvements mirror some of the effects of exercise training. Conversely, blocking CLCF1 activity significantly abolishes these beneficial effects, confirming the crucial role of CLCF1 in mediating the positive effects of exercise on muscle and bone health in male mice. These findings collectively suggest that CLCF1 may contribute to the regulation of age-associated musculoskeletal deterioration, and warrant further investigation into its potential role as a modulator of musculoskeletal health during aging.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Tagliaferri, C., Wittrant, Y., Davicco, M. J., Walrand, S. & Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev.21, 55–70 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

