Safety and tolerability of a novel monoclonal antibody cocktail for rabies post-exposure prophylaxis
- PMID: 40399323
- PMCID: PMC12095772
- DOI: 10.1038/s41541-025-01109-w
Safety and tolerability of a novel monoclonal antibody cocktail for rabies post-exposure prophylaxis
Abstract
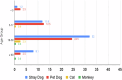
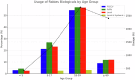
Rabies is one of the most lethal viral zoonoses, particularly in low- and middle-income countries where access to post-exposure prophylaxis is limited. Conventional post-exposure prophylaxis relies on rabies immune globulin and vaccination. This study evaluated the safety of a monoclonal antibody cocktail, docaravimab and miromavimab, in 159 patients with severe animal bites. The cocktail was administered locally to 94.3 percent of participants and both locally and systemically to 5.7 percent. Adverse events were reported in 10.7 percent of cases, predominantly mild and local, with no systemic or severe reactions observed. No cases of rabies occurred during six months of follow-up. These findings suggest that the monoclonal antibody cocktail has a favorable safety profile, potentially serving as a viable alternative to traditional rabies immune globulin in post-exposure prophylaxis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- WHO Expert Consultation on Rabies. Third report, technical report series1012. Geneva: World Health Organization; (2018).
-
- World Health Organization. WHO South East Asia Region: Strategic framework for elimination of human rabies transmitted by dogs in the South–East Asia region. Regional Office for South East Asia, World Health Organization; (2012).
-
- Sudarshan et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J. Infect. Dis.11, 29–35 (2007). - PubMed
LinkOut - more resources
Full Text Sources

