Hemoperfusion with Seraph-100 in septic patients removes pathogens and improves clinical outcomes
- PMID: 40399373
- PMCID: PMC12095501
- DOI: 10.1038/s41598-025-01280-z
Hemoperfusion with Seraph-100 in septic patients removes pathogens and improves clinical outcomes
Abstract
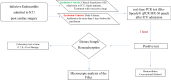
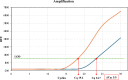
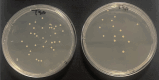
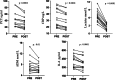
Hemoperfusion (HP) represents a treatment option for sepsis. This study evaluated Seraph-100 in septic patients admitted to the intensive care unit (ICU) after cardiac surgery due to infective endocarditis (IE). Thirteen septic patients were enrolled and treated by Seraph-100 hemoperfusion. Fiftenne patients, not treated by HP, represented a control group. Pathogens were assessed before (T0) and after 4 h of HP treatment (T4). The difference between the two- quantification cycle (Cq) values (T0 and T4), namely ∆Cq at the polymerase chain reaction, was a surrogate marker of pathogen removal. The bacterial load decreased after Seraph-100 HP, with a mean ∆Cq values of 4.6 ± 2.4, as corroborated by conventional haemoculture's results. Field Emission Scanning Electron Microscopy analyses confirm the Seraph' adsorptive properties. Procalcitonin, C reactive protein and lactates significantly decreased, with a reduced ICU stay in the Seraph group. After HP, only 15% of patients had AKI requiring renal replacement therapy (RRT), significantly lower than that found in the control group (40%). The Seraph-100 HP induces a decrease of vasopressor doses, a hemodynamic stability and a reduction of AKI and RRT, improving the clinical course, reflected as a reduced ICU stay.
Keywords: AKI; Hemoperfusion; ICU stay; Sepsis; Seraph-100.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare no competing interests. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University of Messina (Protocol code 41–24) for studies involving humans. Consent for publication: Informed consent was obtained from all subjects involved in the study.
Figures


References
-
- Rhodes, A. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med.43, 304–377 (2017). - PubMed
-
- Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med.49, e1063–e1071 (2021). - PubMed
-
- Kumar, A. et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest136, 1237–1248 (2009). - PubMed
-
- Ferrer, R. et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med.42, 1749–1755 (2014). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

