LXR pathway drives hormonal response intensity in polycystic ovary syndrome
- PMID: 40399491
- PMCID: PMC12254376
- DOI: 10.1038/s44321-025-00251-1
LXR pathway drives hormonal response intensity in polycystic ovary syndrome
Abstract
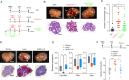
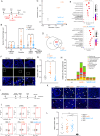
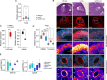
Gonadotropin injections used to stimulate oocyte production during assisted reproductive technology (ART) procedures are associated with the risk of an abnormal response in predisposed patients suffering polycystic ovary syndrome (PCOS). Liver X receptors (LXR) pathway has been identified as key regulators during this process. This study explores the integration of the hormonal signals, cellular networks and molecular mechanisms linking sterol signaling with inflammation and immune infiltration. Pharmacological activation of LXR in a wild-type context protects against gonadotropin hyperstimulation mirroring the effect observed in LXR-deficient mice. Ovarian stimulation leads to immune cell infiltration orchestrated by granulosa cells in absence of LXR, resulting in an altered granulosa cell response to gonadotropin and enhanced inflammation. LXR controls inflammasome activity by regulating Thioredoxin Interacting Protein (TXNIP) gene expression in mural granulosa cells, thereby modulating IL1β production. This immune cell infiltration persists throughout ovulation in PCOS patients and is observed in cumulus oocytes complexes, highlighting the pivotal role of LXR path in regulating inflammatory processes during hormonal stimulation in ART procedures.
Keywords: Granulosa Cells; Hormonal Stimulation; Inflammasome; Liver X Receptors; Polycystic Ovary Syndrome.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures




References
-
- Barañao JL, Hammond JM (1986) FSH increases the synthesis and stores of cholesterol in porcine granulosa cells. Mol Cell Endocrinol 44:227–236 - PubMed
-
- Campbell KL (1979) Ovarian granulosa cells isolated with EGTA and hypertonic sucrose: cellular integrity and function. Biol Reprod 21:773–786 - PubMed
-
- Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P (2003) Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell 12:805–816 - PubMed
-
- Chuderland D, Ben-Ami I, Kaplan-Kraicer R, Grossman H, Ron-El R, Shalgi R (2013) The role of pigment epithelium-derived factor in the pathophysiology and treatment of ovarian hyperstimulation syndrome in mice. J Clin Endocrinol Metab 98:E258–E266 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

