The Potential Impact of Increased Recombinant Zoster Vaccine Uptake in Older Adults Worldwide
- PMID: 40399558
- PMCID: PMC12151947
- DOI: 10.1007/s40121-025-01161-y
The Potential Impact of Increased Recombinant Zoster Vaccine Uptake in Older Adults Worldwide
Abstract
Introduction: Herpes zoster (HZ, Shingles) is a vaccine-preventable viral disease impacting patients' quality of life owing to pain and rash. An estimated 15 million HZ cases occur annually in individuals aged ≥ 50 years worldwide. Recombinant zoster vaccine (RZV) is effective in protecting against HZ. This is the first study evaluating the potential incremental public health benefits in terms of HZ cases averted worldwide by vaccinating adults aged ≥ 50 years with RZV.
Methods: A previously published static multi-cohort Markov model with an annual cycle length and lifetime horizon was used for all analyses. Demographic data depicting populations on 31 December 2023, and age-sex specific mortality rates by region were sourced from United Nations (2022). HZ incidence rates were informed from a recent meta-regression analysis of global HZ burden (Asia, Europe, Northern America, Oceania, and worldwide). RZV efficacy and waning modelling was based on 11-year clinical trial follow-up data [NCT02723773].
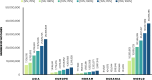
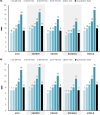
Results: Assuming 70% second-dose compliance in the general population aged ≥ 50 years worldwide, increased RZV uptake by 5% reduced the number of expected HZ cases by > 10 million over the vaccinated cohort's remaining lifetime. More than 5 million of the averted cases were among the cohort vaccinated at ages 50-59 years. Numbers needed to vaccinate (NNV) to avert one HZ case worldwide ranged from 9 at 50-59 years to 18 at ≥ 80 years-of-age, with an overall NNV of 10 for the entire cohort aged ≥ 50 years. Variations observed by region and vaccination age reflected varying inputs, i.e., population counts, HZ incidence rates, mortality rates, and vaccine efficacy waning by age.
Conclusions: A modest (5%) increase in absolute RZV uptake worldwide was estimated to avert millions of additional HZ cases. Lower NNVs were observed in younger vaccinated cohorts irrespective of region, outlining the merits of long-term protection afforded by RZV, and suggesting that earlier vaccination with RZV may be a more effective public health policy against HZ. Greater numbers of averted HZ cases and lower NNVs estimated at ideal second dose compliance demonstrated the importance of timely series completion.
Keywords: Herpes zoster; Public health impact; Recombinant zoster vaccine.
© 2025. GSK Plc.
Conflict of interest statement
Declarations. Conflict of Interest: Justin Carrico is employed by/holds financial equities in GSK. Nikolaos Giannelos and Desmond Curran were employed by GSK during the time of the study, and hold financial equities in GSK. Sean Matthews was an independent contractor for GSK during the time of the study. Anthony L. Cunningham reports financial support (medical support and article processing charges) for the submitted study. Anthony L. Cunningham reports consulting fees from GSK, Moderna, Seqirus, AbbVie; payment/honoraria for lectures from GSK, Moderna, Healthed; research grants/fellowship from GSK made to his institution and personal support for attending meetings from GSK and Moderna. The authors declare no other financial and non-financial relationships and activities. Ethics Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Owing to this reason an ethics committee approval was not required. The authors confirm that this study was performed in accordance with the ethical standards of the Declaration of Helsinki 1964 and its amendments.
Figures

Similar articles
-
How large could the public health impact of introducing recombinant zoster vaccination for people aged ≥50 years in five Latin American countries be?Hum Vaccin Immunother. 2023 Dec 31;19(1):2164144. doi: 10.1080/21645515.2022.2164144. Epub 2023 Feb 23. Hum Vaccin Immunother. 2023. PMID: 36821856 Free PMC article.
-
Public health impact of herpes zoster vaccination on older adults in Singapore: a modeling study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2348839. doi: 10.1080/21645515.2024.2348839. Epub 2024 May 28. Hum Vaccin Immunother. 2024. PMID: 38804600 Free PMC article.
-
Public health impact of herpes zoster vaccination on older adults in Hong Kong.Hum Vaccin Immunother. 2023 Dec 31;19(1):2176065. doi: 10.1080/21645515.2023.2176065. Epub 2023 Feb 28. Hum Vaccin Immunother. 2023. PMID: 36854447 Free PMC article.
-
An Analysis of Spontaneously Reported Data of Vesicular and Bullous Cutaneous Eruptions Occurring Following Vaccination with the Adjuvanted Recombinant Zoster Vaccine.Drug Saf. 2021 Dec;44(12):1341-1353. doi: 10.1007/s40264-021-01118-3. Epub 2021 Oct 7. Drug Saf. 2021. PMID: 34622421 Free PMC article. Review.
-
Recombinant zoster vaccine in immunocompetent and immunocompromised adults: A review of clinical studies.Hum Vaccin Immunother. 2023 Dec 15;19(3):2278362. doi: 10.1080/21645515.2023.2278362. Epub 2023 Nov 15. Hum Vaccin Immunother. 2023. PMID: 37965770 Free PMC article. Review.
References
-
- Schmader K. Herpes zoster. Ann Intern Med. 2018;169(3):ITC17-32. 10.7326/aitc201808070. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

