Bat organoids reveal antiviral responses at epithelial surfaces
- PMID: 40399606
- PMCID: PMC12133567
- DOI: 10.1038/s41590-025-02155-1
Bat organoids reveal antiviral responses at epithelial surfaces
Abstract
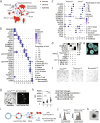
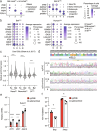
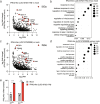
Bats can host viruses of pandemic concern without developing disease. The mechanisms underlying their exceptional resilience to viral infections are largely unresolved, necessitating the development of physiologically relevant and genetically tractable research models. Here, we developed respiratory and intestinal organoids that recapitulated the cellular diversity of the in vivo epithelium present in Rousettus aegyptiacus, the natural reservoir for the highly pathogenic Marburg virus (MARV). In contrast to human counterparts, bat organoids and mucosal tissue exhibited elevated constitutive expression of innate immune effectors, including type I interferon-ε (IFNε) and IFN-stimulated genes (ISGs). Upon infection with diverse zoonotic viruses, including MARV, bat organoids strongly induced type I and III IFN responses, which conferred robust antiviral protection. Type III IFNλ3 additionally displayed virus-independent self-amplification, acting as an ISG to enhance antiviral immunity. Our organoid platform reveals key features of bat epithelial antiviral immunity that may inform therapeutic strategies for viral disease resilience.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures















References
-
- Irving, A. T., Ahn, M., Goh, G., Anderson, D. E. & Wang, L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature589, 363–370 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

