Meropenem plasma concentrations in critically ill patients treated with the novel multi organ replacement therapy ADVOS
- PMID: 40399611
- PMCID: PMC12460481
- DOI: 10.1007/s15010-025-02554-4
Meropenem plasma concentrations in critically ill patients treated with the novel multi organ replacement therapy ADVOS
Abstract
Background: Optimal dosing of antibiotics in critically ill patients treated with the novel multi organ replacement therapy ADVOS (ADVanced Organ Support) based on albumin dialysis is unclear. This study aims to provide real life data on meropenem plasma concentrations after prolonged infusion in patients treated with ADVOS and a critically ill control group with and without continuous veno-venous hemodiafiltration (CVVHDF).
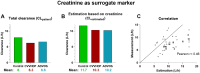
Methods: We retrospectively analyzed plasma concentrations of meropenem obtained as part of our standard of care therapeutic drug monitoring in the intensive care unit. Meropenem was administered as a prolonged infusion over 3 h. We measured peak and trough levels, pre-and post-filter levels of meropenem using high performance liquid chromatography. We calculated the meropenem clearance and compared the measured clearance with predicted clearance based on creatinine, calculated by the MeroEasy tool.
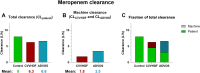
Results: In total, 159 measurements across 16 patients were analyzed. Meropenem trough concentrations were highest in the CVVHDF group with a median of 23.5 mg/L, followed by the ADVOS (median 9.3 mg/L) and control group (median 7.6 mg/L). No trough levels were below the lower limit of 2 mg/L in the CVVHDF and ADVOS groups. Meropenem machine clearance by CVVHDF was calculated to be 1.8 (± 0.5) L/h and 3.5 (± 1) L/h for ADVOS.
Conclusion: Our results suggest that ADVOS treatment in critically ill patients receiving a high dose meropenem regimen (2 g IV q8h) does not lead to underdosing. Some trough values were even within potentially toxic levels, especially in the CVVHDF group, highlighting the importance of therapeutic drug monitoring.
Keywords: ADVOS; Calculated vs. measured values; Meropenem; Multi organ replacement therapy; TDM; Therapeutic drug monitoring.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. AP was employed by ADVITOS GmbH at the time of writing this manuscript. DT, SN and OF received lecture fees by ADVITOS GmbH. Ethical approval and consent to participate: Ethics approval was obtained (EK 24–066-VK), consent to participate is not applicable since we only performed a retrospective data analysis.
Figures

References
-
- Steffens NA, Zimmermann ES, Nichelle SM, Brucker N. Meropenem use and therapeutic drug monitoring in clinical practice: a literature review. J Clin Pharm Ther. 2021;46(3):610–21. - PubMed
-
- Hellinger WC, Brewer NS. Carbapenems and monobactams: imipenem, meropenem, and aztreonam. Mayo Clin Proc. 1999;74(4):420–34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

