Closed-loop vagus nerve stimulation aids recovery from spinal cord injury
- PMID: 40399668
- PMCID: PMC12286844
- DOI: 10.1038/s41586-025-09028-5
Closed-loop vagus nerve stimulation aids recovery from spinal cord injury
Abstract
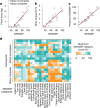
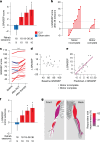
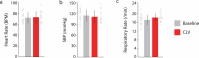
Decades of research have demonstrated that recovery from serious neurological injury will require synergistic therapeutic approaches. Rewiring spared neural circuits after injury is a long-standing goal of neurorehabilitation1,2. We hypothesized that combining intensive, progressive, task-focused training with real-time closed-loop vagus nerve stimulation (CLV) to enhance synaptic plasticity3 could increase strength, expand range of motion and improve hand function in people with chronic, incomplete cervical spinal cord injury. Here we report the results from a prospective, double-blinded, sham-controlled, randomized study combining gamified physical therapy using force and motion sensors to deliver sham or active CLV (ClinicalTrials.gov identifier NCT04288245). After 12 weeks of therapy composed of a miniaturized implant selectively activating the vagus nerve on successful movements, 19 people exhibited a significant beneficial effect on arm and hand strength and the ability to perform activities of daily living. CLV represents a promising therapeutic avenue for people with chronic, incomplete cervical spinal cord injury.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.P.K. has a financial interest in MicroTransponder Inc., which markets VNS therapy for stroke. R.L.R. is the founder and CEO of XNerve, which developed the VNS device used in this study. The other authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

