Sequence diversity lost in early pregnancy
- PMID: 40399685
- PMCID: PMC12176622
- DOI: 10.1038/s41586-025-09031-w
Sequence diversity lost in early pregnancy
Abstract
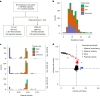
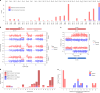
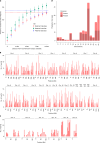
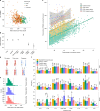
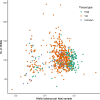
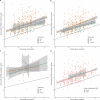
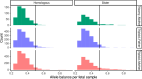
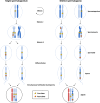
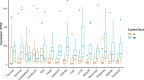
Every generation, the human genome is shuffled during meiosis and a single fertilized egg gives rise to all of the cells of the body1. Meiotic errors leading to chromosomal abnormalities are known causes of pregnancy loss2,3, but genetic aetiologies of euploid pregnancy loss remain largely unexplained4. Here we characterize sequence diversity in early pregnancy loss through whole-genome sequencing of 1,007 fetal samples and 934 parental samples from 467 trios affected by pregnancy loss (fetus, mother and father). Sequenced parental genomes enabled us to determine both the parental and meiotic origins of chromosomal abnormalities, detected in half of our set. It further enabled us to assess de novo mutations on both homologous chromosomes from parents transmitting extra chromosomes, and date them, revealing that 6.6% of maternal mutations occurred before sister chromatid formation in fetal oocytes. We find a similar number of de novo mutations in the trios affected by pregnancy loss as in 9,651 adult trios, but three times the number of pathogenic small (<50 bp) sequence variant genotypes in the loss cases compared with adults. Overall, our findings indicate that around 1 in 136 pregnancies is lost due to a pathogenic small sequence variant genotype in the fetus. Our results highlight the vast sequence diversity that is lost in early pregnancy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors affiliated with deCODE genetics/Amgen declare competing financial interests as employees.
Figures

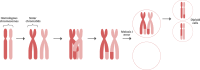




References
-
- Gruhn, J. R. & Hoffmann, E. R. Errors of the egg: the establishment and progression of human aneuploidy research in the maternal germline. Annu. Rev. Genet.56, 369–390 (2022). - PubMed
-
- Sahoo, T. et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet. Med.19, 83–89 (2017). - PubMed
-
- Finley, J. et al. The genomic basis of sporadic and recurrent pregnancy loss: a comprehensive in-depth analysis of 24,900 miscarriages. Reprod. BioMed. Online45, 125–134 (2022). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

