Pharmacokinetics of Ivacaftor, Tezacaftor, Elexacaftor, and Lumacaftor in Special Cystic Fibrosis Populations: A Systematic Review
- PMID: 40399734
- PMCID: PMC12185575
- DOI: 10.1007/s40262-025-01507-2
Pharmacokinetics of Ivacaftor, Tezacaftor, Elexacaftor, and Lumacaftor in Special Cystic Fibrosis Populations: A Systematic Review
Abstract
Background and objective: Following the development of cystic fibrosis transmembrane conductance regulator (CFTR) modulators (ivacaftor, tezacaftor, elexacaftor, and lumacaftor), the prognosis for people diagnosed with cystic fibrosis (pwCF) has improved. Understanding the pharmacokinetics (PK) of CFTR modulators is crucial to provide optimal care, particularly in special cystic fibrosis (CF) populations such as pwCF with hepatic impairment, pancreatic insufficiency, those who are pregnant or lactating, or who are children. We aim to provide an overview of the PK of CFTR modulators in these populations.
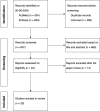
Methods: A systematic literature search was carried out in PubMed and Embase on 20 June 2024. Studies were considered relevant when information on PK or exposure of CFTR-modulating drugs was available.
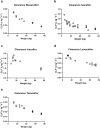
Results: PwCF with mild/moderate hepatic impairment do not exhibit substantially higher exposure to CFTR modulators compared with those without liver involvement or healthy individuals. Similarly, exocrine pancreatic insufficiency has no effect on the PK of CFTR modulators in adult pwCF. In contrast, pediatric pwCF are exposed to higher levels of CFTR modulators relative to adults, as children receive higher weight-based doses (mg/kg) to ensure equivalent therapeutic efficacy.
Conclusions: The PK of CFTR modulators have been more extensively studied in adults, pwCF with mild/moderate hepatic impairment, and children. However, ensuring adequate dosing remains challenging. Knowledge gaps persist for adults with severe hepatic impairment (Child-Pugh Class C), children with CF-induced hepatic impairment, and pregnant or lactating pwCF. Future research addressing these gaps, through incorporating routine clinical data, is crucial for improving clinical guidelines and optimizing dosing regimens, thereby advancing towards evidence-based utilization of CFTR modulators.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. Competing interests: Femke A. Elzinga, Onno H. Akkerman, Bart L. Rottier, Hester van der Vaart, and Daan J. Touw declare that they have no financial or nonfinancial interests that are directly or indirectly related to the work submitted for publication. Paola Mian acknowledges receiving grant support from Vrienden Beatrix Kinderziekenhuis, unrelated to the submitted work. Gerard H. Koppelman discloses grant support from the Netherlands Lung Foundation, ZonMW (Vici grant), Ubbo Emmius Foundation, Teva the Netherlands, Vertex, and GSK, all outside the submitted work (money to institution); his institution received financial support for consultancy or invited presentations from Astra Zeneca, Sanofi, and Boehringer Ingelhei, outside the submitted work. Paul Malik is a full-time employee of Ionis Pharmaceuticals Inc. and may hold stock or stock options. Conflict of interest: Not applicable. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: Data can be accessed upon reasonable request through the corresponding author. Code availability: Not applicable. Author contributions: F.E. contributed to the literature search, data extraction, data analysis, and the writing of the initial draft of the manuscript. P.Ma. contributed to the conceptualization of the study, data analysis, and the writing and revision of the manuscript. O.A., B.R., and H.V. contributed to the revision of the manuscript. G.K. and D.T. contributed to the conceptualization of the study and reviewing the manuscript. P.Mi. contributed to the conceptualization of the study, literature search, data extraction, data analysis, and the writing and revision of the manuscript.
Figures
References
-
- Purkayastha D, Agtarap K, Wong K, Pereira O, Co J, Pakhale S, Kanji S. Drug–drug interactions with CFTR modulator therapy in cystic fibrosis: focus on Trikafta®/Kaftrio®. J Cyst Fibrosis. 2023;22(3):478–83. 10.1016/j.jcf.2023.01.005. - PubMed
-
- Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3(74):74ra24. 10.1126/scitranslmed.3001868. - PMC - PubMed
-
- Mergiotti M, Murabito A, Prono G, Ghigo A. CFTR modulator therapy for rare CFTR mutants. J Respir Med. 2022;2(2):59–76. 10.3390/jor2020005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

