Impact of SGLT2-inhibitors on acute kidney injury in diabetic patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI)
- PMID: 40399991
- PMCID: PMC12096646
- DOI: 10.1186/s12933-025-02773-x
Impact of SGLT2-inhibitors on acute kidney injury in diabetic patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI)
Abstract
Background: Acute kidney injury (AKI) following transcatheter aortic valve implantation (TAVI) is associated with significantly worse outcomes, leading to increased short- and long-term mortality. We sought to evaluate the impact of sodium-glucose cotransporter 2 inhibitors (SGLT2i) on the risk of AKI in patients with type 2 diabetes mellitus (T2DM) and severe aortic stenosis (AS) undergoing TAVI.
Methods: Multicenter international registry of consecutive T2DM patients with severe AS undergoing TAVI between 2021 and 2024. The study population was stratified by the presence of chronic kidney disease (CKD), defined according to the KDIGO guideline, and anti-diabetic therapy at hospital admission (SGLT2i versus no-SGLT2i users). AKI was defined according to the Valve Academy Research Consortium 3 (VARC-3) criteria.
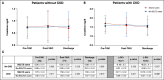
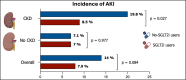
Results: The study population consisted of 514 patients stratified into those without CKD (n = 226, 44%), of whom 43 (19%) were treated with SGLT2i, and 288 (56%) with CKD, of whom 71 (24.7%) were on SGLT2i treatment. The median age was 81 [77-84] years, and 60.1% were males. SGLT2i use did not impact renal function in patients without CKD, with AKI occurring in 7.1% of the cases, regardless of SGLT2i use. Among CKD patients, AKI occurred more frequently in no-SGLT2i users compared to those receiving SGLT2i (19.8% versus 8.5%, p = 0.027), with a significant increase in post-TAVI and discharge serum creatinine values for no-SGLT2i users (p = 0.001 after TAVI and p < 0.001 at hospital discharge). Only in the CKD group, the use of SGLT2i was identified as an independent predictor of a lower rate of AKI (OR 0.70, 95%CI 0.42-0.91, p = 0.014). Patients who developed AKI had a higher incidence of major adverse cardiovascular events during follow-up, regardless of CKD (p < 0.025 for both groups).
Conclusion: In diabetic patients with CKD undergoing TAVI, SGLT2i therapy was associated with a lower occurrence of AKI compared to those not treated with SGLT2i, suggesting a potential nephroprotective effect in this high-risk population.
Keywords: Acute kidney injury; Aortic stenosis; Chronic kidney disease; SGLT2i; Transcatheter aortic valve implantation (TAVI).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The present study was conducted according to the principles of the Declaration of Helsinki; all patients were informed about their participation in the registry and provided informed consent for the anonymous publication of scientific data. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Statement of guarantor: E.B. is the guarantor of the research and, as such, had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Permissions Information: The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Figures

References
-
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. - PubMed
-
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. - PubMed
-
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. - PubMed
-
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

