Mechanism of LINC01018/miR-182-5p/Rab27B in the immune escape through PD-L1-mediated CD8+ T cell suppression in glioma
- PMID: 40399992
- PMCID: PMC12093642
- DOI: 10.1186/s13062-025-00651-w
Mechanism of LINC01018/miR-182-5p/Rab27B in the immune escape through PD-L1-mediated CD8+ T cell suppression in glioma
Abstract
Background: Glioma is a malignant tumor associated with poorer prognosis. This study aims to elucidate the mechanism of LINC01018/miR-182-5p/Rab27B axis in PD-L1-mediated CD8+ T cell suppression in the progression of gliomas.
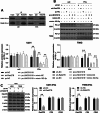
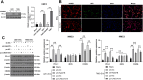
Methods: LINC01018, miR-182-5p, and Rab27B expression levels in glioblastoma tissues were measured. The proportion of infiltrating macrophages and monocytes and CD8+ T cell function were assessed. The relationship between miR-182-5p and Rab27B was analyzed. Glioma cell activity, invasion, and migration were measured. The expression of E-cadherin, N-cadherin, Vimentin, PD-L1, iNOS, and CD206 was determined. Glioma cell-derived EVs were isolated, and the co-localization of Rab27B and PD-L1 and the binding of Rab27B to PD-L1 were analyzed. The endocytosis of EVs by microglia was assayed. The impact of LINC01018/miR-182-5p/Rab27B on glioma growth was observed. The function of macrophages and CD8+ T cells in tumors was analyzed.
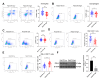
Results: Rab27B was downregulated, and infiltrating macrophages and monocytes were increased in glioblastoma. miR-182-5p inhibited Rab27B expression. Rab27B knockdown reverses the inhibitory effect of LINC01018 overexpression on glioma cell growth. Glioma cells-derived EVs with low Rab27B expression carried more PD-L1 to increase PD-L1 expression and M2 polarization in microglia. LINC01018 overexpression reduced macrophages in orthotopic tumors. CD8+ T cell numbers showed no significant difference, but TIM-3 increased and IFN-γ decreased. miR-182-5p inhibition enhanced the therapeutic effect of anti-PD-L1, which was reversed after glioma cell-derived EVs.
Conclusion: LINC01018 promotes PD-L1-mediated CD8+ T cell suppression via the miR-182-5p/Rab27B axis in glioma cell-derived EVs, thereby contributing to immune escape in gliomas.
Keywords: EVs; Glioma; Immune escape; LINC01018; Rab27B; miR-182-5p.
© 2025. The Author(s).
Conflict of interest statement
Declaration. Ethics approval and consent to participate: The study protocol adhered to the ethical guidelines of ethics committee of Guangzhou Institute of Cancer Research, the Affiliated Cancer Hospital, Guangzhou Medical University and the Declaration of Helsinki. All animal experiments were approved by the ethics committee of Guangzhou Institute of Cancer Research, the Affiliated Cancer Hospital, Guangzhou Medical University and conducted in accordance with the Guide for the Care and Use of Laboratory Animals [16] Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Long non-coding RNA LINC01018 inhibits human glioma cell proliferation and metastasis by directly targeting miRNA-182-5p.J Neurooncol. 2022 Oct;160(1):67-78. doi: 10.1007/s11060-022-04113-5. Epub 2022 Sep 12. J Neurooncol. 2022. PMID: 36094613
-
miR-186-5p Down-Regulates PD-L1 Level in Acute Myeloid Leukemia Cells and Inhibits Tumorigenesis and Immune Escape.J Biochem Mol Toxicol. 2025 May;39(5):e70278. doi: 10.1002/jbt.70278. J Biochem Mol Toxicol. 2025. PMID: 40285500
-
miR-194-5p down-regulates tumor cell PD-L1 expression and promotes anti-tumor immunity in pancreatic cancer.Int Immunopharmacol. 2021 Aug;97:107822. doi: 10.1016/j.intimp.2021.107822. Epub 2021 Jun 4. Int Immunopharmacol. 2021. PMID: 34098485
-
LncRNA LINC01018/miR-942-5p/KNG1 axis regulates the malignant development of glioma in vitro and in vivo.CNS Neurosci Ther. 2023 Feb;29(2):691-711. doi: 10.1111/cns.14053. Epub 2022 Dec 22. CNS Neurosci Ther. 2023. PMID: 36550594 Free PMC article.
-
A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology.Front Immunol. 2021 Sep 17;12:734956. doi: 10.3389/fimmu.2021.734956. eCollection 2021. Front Immunol. 2021. PMID: 34603316 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

