Effectiveness and Safety of a Second JAK Inhibitor in Ulcerative Colitis: The J2J Multicentre Study
- PMID: 40400411
- PMCID: PMC12287878
- DOI: 10.1111/apt.70199
Effectiveness and Safety of a Second JAK Inhibitor in Ulcerative Colitis: The J2J Multicentre Study
Abstract
Background: While three Janus kinase inhibitors (JAKi) have demonstrated efficacy in ulcerative colitis (UC), scarce data exist regarding JAKi intraclass switching.
Aim: To evaluate the effectiveness and safety of a second JAK inhibitor in UC.
Methods: This was a multicentre, retrospective, observational cohort including patients with moderate to severe UC who received a second-line of JAKi after failure or intolerance of a first. The primary outcome was steroid-free clinical remission (SFCR) at Weeks 8-14, defined as a partial Mayo score of 2 or less with no individual sub-score above 1.
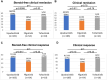
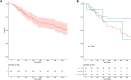
Results: Among the 169 patients from 28 participating centres, 105 received upadacitinib, 54 filgotinib and 10 tofacitinib as a second-line of JAKi. Overall, 81/169 achieved SFCR at Weeks 8-14: 58/105 with upadacitinib, 18/54 with filgotinib and 5/10 with tofacitinib (p = 0.03). In the multivariate analysis, upadacitinib was independently associated with higher odds of SFCR than filgotinib (OR = 3.15, 95% CI [1.52-6.79]). With a median follow-up duration of 96 days, drug persistence at 6 months was 72.8% with upadacitinib, 57.2% with filgotinib and 66.7% with tofacitinib (p = 0.099). 24.3% of patients (41/169) experienced at least one adverse event leading to treatment withdrawal in 9 patients (5%). No cases of death, cancer, or major acute cardiovascular events were reported.
Conclusion: A second-line of JAKi provided clinical remission in about half of patients after induction, and was well tolerated.
Keywords: biologics (IBD); disease‐based; topics; ulcerative colitis.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
M.U. declares counselling, boards or fees for AbbVie, Amgen, Celltrion, Janssen, Lilly and Takeda. R.A. declares counselling, boards or fees for AbbVie, Alphasigma, Amgen, Biogen, Celltrion, Ferring, Janssen, Lilly, MSD, Pfizer, Sandoz and Takeda. M.S. declares boarding or lecture fees for Abbvie, Alfasigma, Amgen, Biogen, Celltrion, Ferring, Janssen, MSD, Pfizer and Takeda. A.B. declares boards or fees for AbbVie, Amgen, Janssen and Takeda. G.C. declares boards for Johnson and Johnson, Lilly; and lectures for Abbvie, Takeda. C.R. has received lecture/consultant fees from Abbvie, Lilly, Janssen, Biogen, Amgen, Takeda and Galapagos. M.F. received lecture and/or consulting fees from Sandoz, Biogen, Amgen, Alfasigma, Galapagos, Ferring, Tillots, Nordic‐Pharma, Celltrion, Janssen, Amgen, Lilly, Pfizer, Janssen, Abbvie, Takeda, Lilly and Gilead. N.H. has served as a consultant/advisory board member to Abbvie, Celltrion, Fresenius Kabi, Janssen and Lilly and as a speaker for Abbvie, Galapagos and Takeda. L.C. declares counselling, boards or fees for AbbVie, Amgen, Celltrion, Johnson & Johnson, Lilly, Pfizer and Takeda. M.C. declares counselling and boards for AbbVie, Janssen, Lilly, Pfizer, MSD and lecture fees for Ferring, Alfasigma, Takeda, Pfizer, Amgen, Lilly, Tillotts and Abbvie. N.R. declares counselling, boards or fees for AbbVie, Amgen, Celltrion, Ferring, Janssen and Takeda. S.V. received lecture and/or consulting fees from AbbVie, Alfasigma, Celltrion, Ferring, Janssen, Lilly and Takeda. P.W. declares lecture fees from Abbvie, Ferring, Takeda, Amgen, Biogen and Janssen. B.C. received lecture and/or consulting fees from AbbVie, Amgen, Celltrion, Ferring, Galapagos, Janssen, Lilly, Nordic Pharma, Pfizer and Takeda. A.A. received consulting fees from Abbvie, Pfizer, Takeda, Adacyte, Sandoz, Tillotts Pharma, Janssen and Sandoz as well as lecture fees and travel accommodations from Abbvie, Janssen, Pfizer, Takeda, Biogen, Fresenius Kabi, Amgen, Adacyte, Ferrin, Biogen and Celltrion. A.N. has received lecture and/or consulting fees from Abbvie, Amgen, Celltrion, Janssen, Alfasigma, Lilly and a grant from MSD‐Avenir. D.L. declares counselling, boards, transports, or fees from Abbvie, Alfasigma, Amgen, Celltrion, Ferring, Janssen, Lilly, Medac, MSD, Pfizer, Sandoz and Takeda. J.K. received lecture fees from Janssen and Lilly, and consulting fees from Roche, Pfizer, Janssen, Abbvie, Takeda, Lilly and Gilead. The other authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
