Mitochondrial fission factor drives an actionable metabolic vulnerability in multiple myeloma
- PMID: 40400476
- PMCID: PMC12485323
- DOI: 10.3324/haematol.2025.287526
Mitochondrial fission factor drives an actionable metabolic vulnerability in multiple myeloma
Abstract
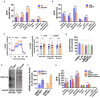
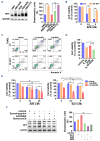
Proliferating multiple myeloma (MM) cells in the bone marrow fluctuate across various metabolic states to resist cancer treatments. Herein, we investigate how mitochondrial dynamics, which control mitochondrial fitness via coordinated fission and fusion events, shape MM cell metabolism impacting growth, survival and drug sensitivity. We identify mitochondrial fission factor (MFF), a pivotal driver of mitochondrial fragmentation, as being highly expressed in MM plasma cells bearing cytogenetic abnormalities predicting poor clinical outcome. In preclinical models, selective inhibition of MFF via multiple RNA-based strategies (short-hairpin RNA, short-interfering RNA or LNA gapmeR antisense oligonucleotides) reduces MM cell growth both in vitro and in vivo, enabling adaptive metabolic responses consistent with the induction of glycolysis and the inhibition of lactate-mediated oxidative phosphorylation. We also demonstrate that lactate supplementation, as well as clinically relevant drugs promoting lactate accumulation, such as AZD3965 and syrosingopine, trigger MFF-dependent metabolic changes, enhancing the sensitivity of MM cells to strategies targeting mitochondrial fission. Finally, we highlight a novel lactate-MFF axis involved in resistance to proteasome inhibitors, and show that combining AZD3965 or syrosingopine with bortezomib results in synergistic anti-MM activity along with MFF downregulation. Collectively, these data point to MFF-dependent mitochondrial fragmentation as a key metabolic hallmark of MM, providing a framework for the development of novel therapeutic strategies targeting mitochondrial dynamics and harnessing the metabolic plasticity of malignant plasma cells.
Figures




References
-
- Alsayyah C, Ozturk O, Cavellini L, Belgareh-Touzé N, Cohen MM. The regulation of mitochondrial homeostasis by the ubiquitin proteasome system. Biochim Biophys Acta. 2020;1861(12):148302. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

