Efficacy and safety of CAR T-cell therapy in patients with primary or secondary CNS lymphoma: A study on behalf of the EBMT and the GoCART coalition
- PMID: 40400509
- PMCID: PMC12093105
- DOI: 10.1002/hem3.70146
Efficacy and safety of CAR T-cell therapy in patients with primary or secondary CNS lymphoma: A study on behalf of the EBMT and the GoCART coalition
Abstract
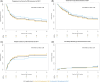
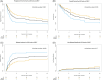
Patients with relapsed or refractory (r/r) primary central nervous system (CNS) lymphoma (PCNSL) or secondary central nervous system (CNS) lymphoma (SCNSL) face a dismal prognosis. They have been excluded from most clinical CAR T-cell trials as investigators feared an increased risk for severe immune effector cell-associated neurotoxicity (ICANS). To investigate the potential of anti-CD19 CAR T-cell therapy (CART) in such patients, we analyzed data of 100 patients with CNS manifestation treated with CART between January 2018 and July 2023 and reported to European Society for Blood and Marrow Transplantation. Median age was 62 years. Of patients, 58% had failed ≥3 treatment lines, and 40% had received autologous stem-cell transplantation before CART. Fifty-nine patients received axicabtagene ciloleucel, 38 patients were treated with tisagenlecleucel, three patients received other products. At the time of CART, 67 patients had active CNS disease. Overall and progression-free survival (PFS) at 24 months were 37% and 28%. Relapse incidence (RI) at 24 months was 59%, whereas non-relapse mortality at 1 year was 7%. Cytokine release syndrome (CRS) and ICANS of any grade occurred in 83% and 42% of patients, respectively. CRS grade 3 occurred in 11 and ICANS grades 3-4 in 17 patients. Two patients died of neurotoxicity. Elevated lactate dehydrogenase was an independent risk factor for RI and PFS (hazard ratio [HR] 2.4, p = 0.003; HR: 1.9, p = 0.016). Patients with ECOG 2-3 had a significantly increased risk for the development of ICANS (HR 2.68, p = 0.002). These data support the implementation of CART as treatment for patients with r/r PCNSL and SCNSL.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Anna Ossami Saidy reports honoraria and travel support from Gilead Kite. Anna Sureda Balari received honoraria from Takeda, BMS/Celgene, MSD, Janssen, Amgen, Novartis, Gilead Kite, Sanofi, Roche, GenMab, AbbVie, Jazz Pharmaceuticals, AstraZeneca, Pierre Fabre, and Menarini; held a consultancy role with Takeda, BMS/Celgene, Novartis, Janssen, Gilead, Sanofi, GenMab, AbbVie, AstraZeneca, and Incyte; participated in Takeda's speaker's bureau; and received research support from Takeda. Ron Ram received honoraria from Kite/Gilead and Novartis. Bastian von Tresckow is an advisor or consultant for Allogene, Amgen, BMS/Celgene, Cerus, Gilead Kite, Incyte, IQVIA, Janssen‐Cilag, Lilly, Merck Sharp & Dohme, Miltenyi, Novartis, Noscendo, Pentixapharm, Pfizer, Pierre Fabre, Qualworld, Regeneron, Roche, SOBI, and Takeda; has received honoraria from AbbVie, AstraZeneca, BMS/Celgene, Gilead Kite, Incyte, Janssen‐Cilag, Lilly, Merck Sharp & Dohme, Novartis, Roche, and Takeda; reports research funding from Esteve (Inst.), Merck Sharp & Dohme (Inst.), Novartis (Inst.), and Takeda (Inst.); and reports travel support from AbbVie, AstraZeneca, Gilead Kite, Janssen‐Cilag, Lilly, Merck Sharp & Dohme, Pierre Fabre, Roche, Takeda, and Novartis. Francis Ayuk received honoraria from AbbVie, Celgene/BMS, Kite Gilead, Janssen, Mallinckrodt/Therakos, Miltenyi Biomedicine, Novartis, Takeda, and Medac; and research funding from Mallinckrodt/Therakos. Wolfgang Bethge received consulting honoraria from Gilead GmbH, Novartis GmbH, Celgene GmbH, AbbVie, and Janssen‐Cilag GmbH; and research funding from Miltenyi Biotec GmbH. Elisabeth Schorb reports receipt of lecture fees from SERB Pharmaceuticals. Friedrich Stölzel reports a scientific advisory role with Pierre Fabre and Servier; received travel support from Medac, Servier, and Johnson & Johnson; and received honoraria from Medscape, Clinigen, and Jazz Pharmaceuticals. Matthias Stelljes has served as a consultant for Pfizer, MSD, BMS, Incyte, Takeda, Astellas, and Amgen; served as a speaker for Pfizer, Medac, MSD, Astellas, Jazz Pharmaceuticals, Amgen, Novartis, Kite/Gilead, Celgene, BMS, AbbVie, and Incyte; received research funding from Pfizer; and travel and accomodation support from Kite/Gilead, Medac, and Pfizer. Marc Wehrli received travel support from Kite/Gilead. Michael Daskalakis received travel and accommodation support from Kite/Gilead, Novartis, Amgen, and Novo Nordisk; and held a consulting or advisory role with Novartis and Alexion Pharma. Andrea Kuhnl received honoraria from Kite/Gilead, BMS, AbbVie, and Roche; and travel support from Kite/Gilead and AstraZeneca. Stephan Fuhrmann received honoraria from BMS/Celgene and Kite/Gilead. Norbert Schmitz received honoraria from Roche; travel grants from BeiGene; owned stock from BMS; and received research grants from Janssen. Catherine Thieblemont reports advisory function and honorarium from Roche, Incyte, Kite/Gilead, Novartis, AstraZeneca, and Takeda. Peter Dreger received honoraria from Gilead Sciences, AbbVie, Bristol Myers Squibb/Celgene, Roche, and BeiGene; reports a consulting or advisory role for Gilead Sciences, AbbVie, BeiGene, Bristol Myers Squibb/Celgene, and Miltenyi Biomedicine; received research funding from RIEMSER; and received travel and accommodation support from BeiGene and Gilead Sciences. Bertram Glass reports a consulting or advisory role for Roche Pharma AG, BMS GmbH & Co. K, Kite, a Gilead company, Novartis, RIEMSER, Jazz Pharmaceuticals, Miltenyi Biotec, Janssen, and AbbVie and received research funding from Roche and RIEMSER. No other potential conflicts of interest were reported.
Figures
References
-
- Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma—a survey of 1693 patients treated in protocols of the German High‐Grade Non‐Hodgkin's Lymphoma Study Group (DSHNHL). Ann Oncol. 2007;18:149‐157. - PubMed
-
- Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B‐cell lymphoma treated with R‐CHOP. J Clin Oncol. 2016;34:3150‐3156. - PubMed
