North American expert consensus on the clinical role of ex vivo lung perfusion (EVLP) with acellular perfusate
- PMID: 40400975
- PMCID: PMC12090176
- DOI: 10.21037/jtd-2024-2069
North American expert consensus on the clinical role of ex vivo lung perfusion (EVLP) with acellular perfusate
Abstract
Background: Ex vivo lung perfusion (EVLP) of donor lungs not otherwise acceptable for transplantation can provide outcomes similar to standard-criteria lung transplantation and has been reported to increase transplant volume by approximately 20% in some transplant centers. Evidence to support decisions about use of EVLP is limited, so expert opinion can be a useful decision aid. This study developed expert consensus recommendations for EVLP with acellular perfusate using a modified Delphi method.
Methods: A panel of 18 physicians with expertise in lung transplantation and EVLP who practice in North America completed three surveys on EVLP: Survey 1 used open-ended questions; Survey 2 used primarily Likert-scale questions; and Survey 3 repeated Survey 2 while providing panelists with the Survey 2 results. A follow-up meeting after Survey 3 probed open questions.
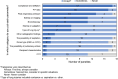
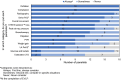
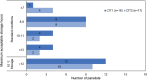
Results: The primary goal for EVLP is expanding the number of donor lungs available for transplant. Lungs that are acceptable after EVLP are equivalent to lungs that met standard criteria initially. Lungs with unclear or marginal quality should be placed on EVLP for evaluation, including lungs received from third party organizations with incomplete or concerning information. Decisions on whether to put lungs on EVLP require nuanced clinical judgement and should consider compliance and deflation, the ratio of PaO2 to fraction of inspired oxygen (P/F ratio), peak inspiratory pressure (PIP), edema on imaging, and bronchoscopy, with additional parameters considered as appropriate if lung quality is unclear. EVLP lungs are appropriate for transplant if all relevant parameters are acceptable and may be appropriate if some parameters are borderline depending on clinical judgment. Decisions about transplanting EVLP lungs should consider radiography, delta PO2, overall movement, STEEN Solution™ loss, bronchoscopy, peak airway pressure, and palpation, along with other parameters as appropriate. Key open areas for research include evidence-based criteria for lung selection and assessment, the role of biomarkers, and enhanced techniques and perfusion solutions. In addition, the role of EVLP is unclear in lungs with pulmonary emboli and lungs procured with normothermic regional perfusion (NRP), as is the maximal duration of cold ischemia time (CIT).
Conclusions: Decisions about EVLP require nuanced consideration of numerous parameters. Expert opinion from this study may help optimize use of EVLP.
Keywords: Lung transplant; acellular perfusate; donor lung evaluation; ex vivo lung perfusion (EVLP).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2069/coif). B.A.W. serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2023 to July 2025. All authors report that the study was sponsored and supported by Lung Bioengineering, Inc. M.B. has received grants from the National Institutes of Health, Congressionally Directed Medical Research Programs, and Cystic Fibrosis Foundation; is a board member for Xylyx Bio, Inc. and the Chief Medical Officer (unpaid, no intellectual property, patents, nor stock) for ART, Inc.; and holds several issued or pending patents related to EVLP and transplantation. C.A.B. has received consulting fees for serving on medical advisory boards for Abbot and Abiomed. A.W.B. has received grants from and is a part-time employee of the Cystic Fibrosis Foundation, received consulting fee from Lung Bioengineering, Inc., and has served as a volunteer on the OPTN Lung Transplantation Committee Region 2 and the OPTN Lung Exception Review Board. M.M.B. has participated on an advisory board for Lung Bioengineering, Inc. and Transmedics, Inc., and has held leadership roles as the UNOS Lung Committee Chair and on the UNOS Multiorgan Committee and UNOS Placement Efficiency Taskforce. M.C. has acted as a consultant for Lung Bioengineering, Inc., Incyte, and Avivo, and is the Chief Scientific Officer for and a shareholder in Traferox Technologies Inc. S.K. is the Chief Medical Officer for Traferox Technologies Inc., a consultant for United Therapeutics Corp., Lung Bioengineering, Inc., CareDx, Abbott, and CSL Behring, and holds pending or issued patents on EVLP technologies and lung diagnostic biomarkers. Z.N.K. is the Chief Executive Officer of ProCure On-Demand, Inc. He holds stock in ProCure On-Demand, Inc. and notes that Lung Bioengineering, Inc. is a minority investor. J.M.M. has consulted for Lung Bioengineering, Inc. (payments made to the Mayo Clinic, his employer). T.K.W.’s institution has received research grants and royalties or licenses from United Therapeutics Corp. He has received consulting fees from Lung Bioengineering, Inc., and has several patents related to EVLP. He is a founder of Traferox Technologies, Inc., and holds stock and stock options in the company. B.A.W. has received grants from the National Institutes of Health and participated on the Clinical Events Committee for Transmedics Inc. K.R.M. has received royalties related to intellectual property from XVIVO; received lecture honoraria from Lung Bioengineering Inc. and XVIVO; and received support for attending meetings from XVIVO; holds pending or issued patents related to lung evaluation and EVLP; has a volunteer leadership role on the UNOS/OPTN Board of Directors; and has stock or stock options in TransMedics, Inc. The authors have no other conflicts of interest to declare.
Figures

Similar articles
-
Porcine lungs perfused with three different flows using the 8-h open-atrium cellular ex vivo lung perfusion technique.Front Bioeng Biotechnol. 2024 Jun 25;12:1357182. doi: 10.3389/fbioe.2024.1357182. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38983601 Free PMC article.
-
Prolonged extracorporeal preservation and evaluation of human lungs with portable normothermic ex vivo perfusion.Clin Transplant. 2020 Mar;34(3):e13801. doi: 10.1111/ctr.13801. Epub 2020 Feb 19. Clin Transplant. 2020. PMID: 31999865
-
Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial.J Heart Lung Transplant. 2017 Jul;36(7):744-753. doi: 10.1016/j.healun.2017.02.011. Epub 2017 Feb 20. J Heart Lung Transplant. 2017. PMID: 28314503 Clinical Trial.
-
Outcomes of marginal donors for lung transplantation after ex vivo lung perfusion: A systematic review and meta-analysis.J Thorac Cardiovasc Surg. 2020 Feb;159(2):720-730.e6. doi: 10.1016/j.jtcvs.2019.07.087. Epub 2019 Aug 25. J Thorac Cardiovasc Surg. 2020. PMID: 31548078
-
The Conversional Efficacy of Ex Vivo Lung Perfusion and Clinical Outcomes in Patients Undergoing Transplantation of Donor Lungs by Ex Vivo Lung Perfusion: A Meta-Analysis.Ann Transplant. 2019 Dec 27;24:647-660. doi: 10.12659/AOT.919242. Ann Transplant. 2019. PMID: 31879416 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
