PRRT 2-Related Epilepsy: From Self-Limited Infantile Epilepsy to Atypical Epilepsy Phenotypes
- PMID: 40401013
- PMCID: PMC12094785
- DOI: 10.1212/NXG.0000000000200267
PRRT 2-Related Epilepsy: From Self-Limited Infantile Epilepsy to Atypical Epilepsy Phenotypes
Abstract
Background and objectives: Pathogenic variants in the PRRT2 gene cause self-limited infantile epilepsy (SeLIE). Recently, atypical epilepsy phenotypes have been described. We explore the phenotypic spectrum of PRRT2-related epilepsy through international collaboration.
Methods: All children with epilepsy and either a pathogenic PRRT2 variant or 16p11.2 microdeletion encompassing the PRRT2 gene were included in this retrospective study. Details related to the epilepsy, comorbidities, genetic results, EEG/neuroimaging findings, and treatments are summarized.
Results: Forty children were identified, and 24 were male (n = 24/40, 60%). The median age at seizure onset was 5 months (IQR 4, 6) (range 2-150 months). Bilateral tonic-clonic (BTC) (n = 22/40, 55%) and focal motor seizures with impaired awareness evolving to BTC seizures (n = 9/40, 23%) were most common. Thirty-six children (n = 36/40, 90%) had pathogenic PRRT2 variants; 3 were homozygous, and 33 were heterozygous. Four children (n = 4/40, 10%) had 16p11.2 microdeletion. SeLIE was most common, diagnosed in 32 children with heterozygous PRRT2 variants (n = 32/33, 97%), 3 children with homozygous PRRT2 variants (n = 3/3, 100%), and 3 children with 16p11.2 microdeletion (n = 3/4, 75%). Atypical phenotypes were observed in 3 children with heterozygous PRRT2 variants; 1 child evolved from SeLIE to infantile epileptic spasms, another developed spike-wave activation in sleep, and 1 developed focal epilepsy in adolescence. Medically refractory genetic generalized epilepsy and intellectual disability were diagnosed in 1 child with a whole-gene PRRT2 deletion and 16p11.2 microdeletion. All children with homozygous PRRT2 variants had SeLIE with movement disorders. Thirty-seven children (n = 37/40, 93%) were treated with antiseizure medications, and sodium channel blockers were effective in most (20/27 responded, 74%). The median age at seizure freedom was 9 months (IQR 3, 10) (range 3-168 months).
Discussion: Pathogenic PRRT2 variants are commonly associated with SeLIE. However, additional epilepsy phenotypes may be observed with heterozygous PRRT2 variants. In individuals with heterozygous PRRT2 variants, corresponding chromosomal microarray may be helpful to assess for concomitant 16p11.2 microdeletion, given the phenotypic overlap between the 2 conditions. Collection of additional cases is needed, however, to better understand the spectrum of epilepsy phenotypes associated with 16p11.2 microdeletion encompassing the PRRT2 gene and homozygous and compound heterozygous PRRT2 variants. Currently, precise genotype-phenotype relationships are lacking.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
M. Komar, J. Sidhu, J. Joseph, A. Kumar, D.J.A. Callen, and R. Mesterman have no disclosures. R. Ramachandrannair received a consulting fee from UCB Canada. K.C. Jones, B.F. Meaney, S. Singh, L. McRae, V. Chau, and S. Sharma have no disclosures. E.J. Donner has received consulting fees from Jazz Pharmaceuticals and UCB. R. Aaron, S. Danda, M. Thomas, L. Saini, G. Costain, S. Yoganathan, and P. Jain have no disclosures. R. Whitney has received consulting fees from Jazz Pharmaceuticals and Takeda Pharmaceuticals in the past. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
Figures

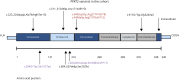
References
-
- DGama AM, Mulhern S, Sheidley BR, et al. Evaluation of the feasibility, diagnostic yield and clinical utility of rapid-genome sequencing in infantile epilepsy (Gene-steps): an international, multicentre, pilot cohort study. Lancet Neurol. 2023;22(9):812-825. doi: 10.1016/S1474-4422(23)00246-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
