Real world study in Italian public hospital with Efgartigimod in patients affected by generalized myasthenia gravis: influence of clinical and serological factors
- PMID: 40401017
- PMCID: PMC12092212
- DOI: 10.3389/fneur.2025.1555068
Real world study in Italian public hospital with Efgartigimod in patients affected by generalized myasthenia gravis: influence of clinical and serological factors
Abstract
Background: Myasthenia gravis (MG) is an autoimmune neuromuscular disorder caused by IgG autoantibodies targeting the neuromuscular junction. Recycling of IgG is mediated by the neonatal Fc receptor (FcRn). Efgartigimod, an Fc fragment of human IgG1, has demonstrated efficacy in MG; however, the clinical characteristics of patients with the highest response remain unclear.
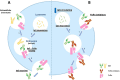
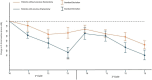
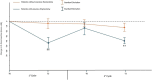
Methods: Twelve patients with AChR-positive generalized MG were treated with two cycles of Efgartigimod over 1 year, and nine patients completed a third cycle. Clinical evaluation was conducted using MG-ADL at four time points and QMG at the beginning and end of each cycle. MG-ADL and QMG scores were further subdivided into ocular (O), bulbar (B), and generalized (G) symptom subdomains, and patients were classified as predominantly ocular (pO), bulbar (pB), or generalized (pG) based on symptom prevalence.
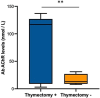
Results: Significant improvements were observed in MG-ADL and QMG from baseline across all symptom subdomains. Baseline AChR antibody levels correlated with MG-ADL improvement (p < 0.04). Thymectomized patients demonstrated superior outcomes, with MG-ADL improving by 62% versus 22% (p < 0.01) and QMG by 45% versus 3.5% (p < 0.01) during the first two cycles. Patients with pO symptoms responded less to therapy, with generalized symptoms contributing most to the minor response.
Discussion: Our findings suggest that patients with high baseline AChR antibody titers, previous thymectomy, and non-ocular symptom predominance respond better to Efgartigimod. These results underscore the need for larger studies to validate these observations and optimize patient selection.
Keywords: AChR; Efgartigimod; Myasthenia Gravis; T cells; Thymectomy.
Copyright © 2025 Sgarzi, Paone, Camera, Agazzi, Mazzoleni, Martorana and Alimonti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

