Valutazione dei tempi di accesso per le nuove entità chimiche in Italia: un’analisi critica del periodo 2018-2024
- PMID: 40401201
- PMCID: PMC12093107
- DOI: 10.33393/grhta.2025.3422
Valutazione dei tempi di accesso per le nuove entità chimiche in Italia: un’analisi critica del periodo 2018-2024
Abstract
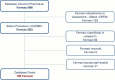
Objective:: This study aims to provide an up-to-date analysis, for new chemical entities on the market, on the timing of pricing and reimbursement (P&R) in Italy, covering the entire period of activity (from September 2018 to January 2024) of the last evaluation Commission (Technical Scientific Committee, CTS, and Price and Reimbursement Committee, CPR).
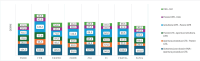
Methods:: The data used in this analysis were obtained from public official websites. The information was systematically collected to investigate the days required to complete the P&R process. The analysis was stratified into indications for rare diseases, orphan designation, innovation assessment, and anatomical therapeutic chemical (ATC) class L. Mann-Whitney U test was used to study the significance of the difference. A p-value < 0.05 was considered significant.
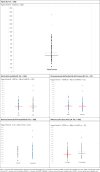
Results:: Overall, 180 procedures were included in the final analysis. The median duration of the entire process, from MAH submission to final Gazette publication, was of 439.5 days. The timelines varied among the analyzed drug classes. Rare disease drugs (n = 78) had a longer timeline than non-rare-disease drugs (n = 102) (462.0 days vs 419.5 days, respectively). Among rare disease procedures, orphan designation was a predictor for time prolongation (orphan drugs, n = 66, 462.0 days vs 443.0 days non-orphan drugs, n = 12). Innovativeness status was associated with a shorter timeframe (-14 days), as was the ATC L classification (-68 days).
Conclusion:: The results indicate a methodological consistency with previous studies, suggesting a continuity of analysis, despite the increase in the complexity of evaluations and the number of drugs treated.
Conflict of interest statement
Conflict of interest: The Authors declare no conflict of interest.
Figures

References
-
- Regolamento (CE) n. 726/2004 del Parlamento europeo e del Consiglio del 31 marzo 2004 che istituisce procedure comunitarie per l'autorizzazione e la sorveglianza dei medicinali per uso umano e veterinario, e che istituisce l'agenzia europea per i medicinali (Testo rilevante ai fini del SEE). [(Accessed December; 2024 )]; Online
-
- EFPIA Patients W.A.I.T. Indicator 2023 Survey (published June 2024): [(Accessed December; 2024 )]; Online
LinkOut - more resources
Full Text Sources

