Fetoplacental extracellular vesicles deliver conceptus-derived antigens to maternal secondary lymphoid tissues for immune recognition
- PMID: 40401522
- PMCID: PMC12128977
- DOI: 10.1172/jci.insight.186335
Fetoplacental extracellular vesicles deliver conceptus-derived antigens to maternal secondary lymphoid tissues for immune recognition
Abstract
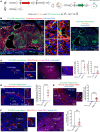
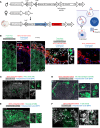
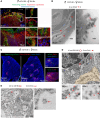
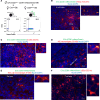
Pregnancy is an immunological paradox where despite a competent maternal immune system, regulatory mechanisms at the fetoplacental interface and maternal secondary lymphoid tissues (SLTs) circumvent rejection of semi-allogeneic concepti. Small extracellular vesicles (sEVs) are a vehicle for intercellular communication; nevertheless, the role of fetoplacental sEVs in transport of antigens to maternal SLTs has not been conclusively demonstrated. Using mice in which the conceptus generates fluoroprobe-tagged sEVs shed by the plasma membrane or released from the endocytic compartment, we show that fetoplacental sEVs are delivered to immune cells in the maternal spleen. Injection of sEVs from placentas of females impregnated with Act-mOVA B6 males elicited suboptimal activation of OVA-specific CD8+ OT-I T cells in virgin females as occurs during pregnancy. Furthermore, when OVA+ concepti were deficient in Rab27a, a protein required for sEV secretion, OT-I cell proliferation in the maternal spleen was decreased. Proteomics analysis revealed that mouse trophoblast sEVs were enriched in antiinflammatory and immunosuppressive mediators. Translational relevance was tested in humanized mice injected using sEVs from cultures of human trophoblasts. Our findings show that sEVs deliver fetoplacental antigens to the mother's SLTs that are recognized by maternal T cells. Alterations of such a mechanism may lead to pregnancy disorders.
Keywords: Antigen; Immunology; Mouse models; Reproductive biology; T cells.
Conflict of interest statement
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

