Safety and Efficacy of Cobalt Chromium Everolimus-Eluting Stents for Treatment of In-Stent Restenosis: An ILUMIEN IV Substudy
- PMID: 40401609
- PMCID: PMC12229119
- DOI: 10.1161/JAHA.124.039482
Safety and Efficacy of Cobalt Chromium Everolimus-Eluting Stents for Treatment of In-Stent Restenosis: An ILUMIEN IV Substudy
Abstract
Background: The optimal management strategy for in-stent restenosis (ISR) remains unclear. We aimed to determine the safety and efficacy of percutaneous coronary intervention with XIENCE cobalt chromium everolimus-eluting stents (EES) for treatment of ISR.
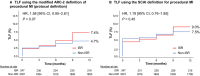
Methods: In the ILUMIEN IV (Optical Coherence Tomography Guided Coronary Stent Implantation Compared to Angiography: A Multicenter Randomized Trial in Percutaneous Coronary Intervention) trial, the 1-year outcomes of all randomized patients with a single diffuse or multifocal single-layer ISR lesion treated with EES were compared with a performance goal. The primary end point was target lesion failure, the composite of cardiac death, target vessel-myocardial infarction, or ischemia-driven target lesion revascularization. Outcomes in patients with a single EES-treated ISR and non-ISR lesion were also compared.
Results: From May 2018 through December 2020, 247 patients with a single ISR lesion were treated with EES. Target lesion failure at 1 year occurred in 18 patients (7.4% [upper 1-sided 97.5% CI, 11.5%]), which was lower than the predefined performance goal of 20% (P<0.001). Compared with non-ISR lesions treated with EES (n=2021), the postpercutaneous coronary intervention minimal stent area by optical coherence tomography was smaller in treated ISR lesions (5.0±1.8 mm2 versus 5.6±1.9 mm2; P<0.001), but minimum stent expansion was similar (78.8±18.0% versus 79.0±16.9%; P=0.87), as was 1-year target lesion failure (7.4% versus 4.7%; hazard ratio, 1.58 [95% CI, 0.95-2.61]; P=0.07).
Conclusions: XIENCE EES was safe and effective for treatment of single-layer ISR. Compared with non-ISR lesions, ISR lesions treated with EES had a smaller postpercutaneous coronary intervention minimal stent area although stent expansion and 1-year target lesion failure rates were not different.
Registration: URL: https://clinicaltrials.gov/; Unique identifier: NCT03507777.
Keywords: drug‐eluting stent; in‐stent restenosis; percutaneous coronary intervention.
Conflict of interest statement
Z.A. Ali reports institutional grants from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems Inc, Medtronic, Opsens Medical, Philips. Shockwave Medical; personal fees from Amgen, Astra Zeneca, Boston Scientific; equity from Elucid, Lifelink, Spectrawave, Shockwave and Vital Connect. U. Landmesser reports receiving lecture fees and advisory fees from AstraZeneca, Amgen, and Sanofi, and grant support, lecture fees, or advisory fees from Abbott and Novartis. A. Maehara consultant for Boston Scientific, received speaker honoraria from Nipro, and is on the adversary board of SpectraWAVE. M. Matsumura is a consultant for TERUMO and Boston Scientific. E. Shlofmitz has been a consultant to Abbott Vascular, ACIST Medical, Boston Scientific, Gentuity, Philips, RadiAction, Shockwave Medical and Terumo. Y. S. Abdelwahed reports receiving lecture fees and advisory fees from Boston Scientific and Shockwave. P. Canova is a consultant for Abbott Vascular and Boston Scientific; and has received grants from Amarin Pharma Inc, Amgen, Bristol Myers Squibb Company, Daiichi‐Sankyo, Sanofi, and Terumo. N. Gonzalo has received a research grant from Abbott; and consultancy and speaker fees from Abbott, Boston Scientific, Shockwave Medical, Abiomed, and Philips. K.N. Fall is a consultant for Abbott, Boston Scientific, and Conavi. R.J. McGreevy, R.W. McNutt, H. Nie, J. Wang and J. Buccola are employees of Abbott Vascular. G.W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, and Abbott; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, and Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, and Valfix, Xenter. Dr Stone's daughter is an employee at IQVIA. Institutional disclosure: Dr Stone's employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems, Inc, Phillips, Biosense Webster, Shockwave, Vascular Dynamics, Pulnovo, and V‐Wave. The other authors report nothing to disclose relevant to the article.
Figures

References
-
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071 - DOI - PubMed
-
- Lawton JS, Tamis‐Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e18–e114. doi: 10.1161/CIR.0000000000001038 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

