Interaction of Body Mass Index and Glycemic Status on Cardiovascular Outcomes in Patients With Cancer Treated With Anthracyclines
- PMID: 40401613
- PMCID: PMC12229114
- DOI: 10.1161/JAHA.124.040876
Interaction of Body Mass Index and Glycemic Status on Cardiovascular Outcomes in Patients With Cancer Treated With Anthracyclines
Abstract
Background: Anthracycline-based chemotherapy is a vital treatment for various cancers but carries notable risks of cardiotoxicity. This study aimed to assess how different body mass index values and glycemic status influence the risk of major adverse cardiovascular events (MACE) in chemotherapy-naïve adult patients with cancer treated with anthracyclines.
Methods: This retrospective cohort included 11 393 chemotherapy-naïve patients who initiated anthracycline-based chemotherapy between 2000 and 2019. Follow-up began from the first anthracycline dose. Body mass index was categorized as underweight/normal weight (<25 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2). Glycemic status was classified as normoglycemic or diabetes/prediabetes (diabetes: hemoglobin A1c ≥6.5% or fasting glucose ≥126 mg/dL; prediabetes: hemoglobin A1c 5.7%-6.4% or fasting glucose 100-125 mg/dL).
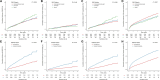
Results: Over a median follow-up of 8.7 years, 985 (8.64%) patients experienced MACE. Obesity was significantly associated with an increased risk of MACE (hazard ratio [HR], 1.38 [95% CI, 1.10-1.73], reference: underweight/normal weight) and heart failure hospitalization, and diabetes/prediabetes also significantly predicted MACE (HR, 1.28 [95% CI, 1.10-1.50], reference: normoglycemic). Notably, overweight (HR, 0.85 [95% CI, 0.80-0.91]) and obesity (HR, 0.85 [95% CI, 0.74-0.96]) were associated with lower risk of all-cause mortality. Joint analysis revealed that patients with both obesity and diabetes/prediabetes had the highest risk of MACE (HR, 1.74 [95% CI, 1.28-2.37]) and heart failure hospitalization (HR, 1.99 [95% CI, 1.41-2.81]).
Conclusions: In patients with cancer undergoing anthracycline-based chemotherapy, both body mass index and glycemic status significantly affect cardiovascular risks, with the highest risk observed in those with concurrent obesity and diabetes/prediabetes, emphasizing the need for tailored risk assessment and management.
Keywords: anthracycline‐based chemotherapy; body mass index; cardiotoxicity; cardiovascular outcomes; glycemic status; heart failure; mortality.
Conflict of interest statement
None.
Figures

References
-
- Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

