Mapping Glial Autophagy Dynamics in an Amyotrophic Lateral Sclerosis Mouse Model Reveals Microglia and Astrocyte Autophagy Dysfunction
- PMID: 40401739
- PMCID: PMC12313006
- DOI: 10.1002/glia.70045
Mapping Glial Autophagy Dynamics in an Amyotrophic Lateral Sclerosis Mouse Model Reveals Microglia and Astrocyte Autophagy Dysfunction
Abstract
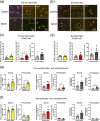
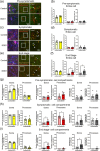
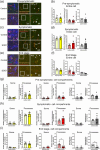
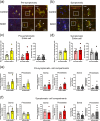
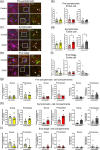
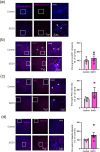
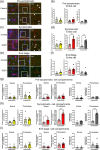
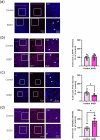
Amyotrophic lateral sclerosis (ALS) is defined by motor neuron death. However, recent research has identified non-cell-autonomous mechanisms, with significant involvement of glia in disease progression. We link previous observations of intracellular protein aggregates in glia to the autophagy pathway, the primary mediator of intracellular degradation of large protein aggregates. While dysfunctional autophagy is reported in ALS motor neurons, pre-clinical and clinical outcomes of autophagy modulators have been inconsistent, indicating the need for a nuanced understanding of autophagy dynamics across CNS cell types and ALS-affected regions. We hypothesized that glial autophagy is defective in ALS, with glial-type-specific dysfunction. To investigate in vivo autophagy dynamics, we intercrossed SOD1G93A mice with transgenic RFP-EGFP-LC3 autophagy reporter mice, enabling the quantification of autophagy degradation, termed flux. Investigation of autophagy dynamics in SOD1 oligodendrocytes, microglia, and astrocytes at key disease stages uncovered useful insights. While oligodendrocytes seemed to mount effective compensatory autophagic responses to combat mutant SOD1, significantly increased autophagy flux was observed in symptomatic spinal microglia and astrocytes in comparison to controls. Symptomatic SOD1 astrocytes displayed greater autophagy dysfunction compared to microglia, with subcellular analysis revealing cell compartment-specific, transient autophagy defects that returned to control levels by end stage. Interestingly, spinal glia showed more pronounced and earlier autophagy dysfunction compared to motor cortex glia, where autophagy dysfunction emerged later in disease end stage, aligning with greater spinal cord pathology reported in this model. Our results suggest that cell-type- and time-specific targeting might be essential when developing autophagy therapeutics for ALS, with prioritization of astrocytic autophagy modulation.
Keywords: ALS; SOD1; SOD1G93A; astrocytes; autophagy; microglia; oligodendrocytes.
© 2025 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Arai, T. , Hasegawa M., Akiyama H., et al. 2006. “TDP‐43 Is a Component of Ubiquitin‐Positive Tau‐Negative Inclusions in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis.” Biochemical and Biophysical Research Communications 351, no. 3: 602–611. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

