European Stroke Organisation (ESO) and European Association of Neurosurgical Societies (EANS) guideline on stroke due to spontaneous intracerebral haemorrhage
- PMID: 40401775
- PMCID: PMC12098356
- DOI: 10.1177/23969873251340815
European Stroke Organisation (ESO) and European Association of Neurosurgical Societies (EANS) guideline on stroke due to spontaneous intracerebral haemorrhage
Abstract
Spontaneous (non-traumatic) intracerebral haemorrhage (ICH) affects ~3.4 million people worldwide each year, causing ~2.8 million deaths. Many randomised controlled trials and high-quality observational studies have added to the evidence base for the management of people with ICH since the last European Stroke Organisation (ESO) guidelines for the management of spontaneous ICH were published in 2014, so we updated the ESO guideline. This guideline update was guided by the European Stroke Organisation (ESO) standard operating procedures for guidelines and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework, in collaboration with the European Association of Neurosurgical Societies (EANS). We identified 37 Population, Intervention, Comparator, Outcome (PICO) questions and prioritised clinical outcomes. We conducted systematic literature searches, tailored to each PICO, seeking randomised controlled trials (RCT) - or observational studies when RCTs were not appropriate, or not available - that investigated interventions to improve clinical outcomes. A group of co-authors allocated to each PICO screened titles, abstracts, and full texts and extracted data from included studies. A methodologist conducted study-level meta-analyses and created summaries of findings tables. The same group of co-authors graded the quality of evidence, and drafted recommendations that were reviewed, revised and approved by the entire group. When there was insufficient evidence to make a recommendation, each group of co-authors drafted an expert consensus statement, which was reviewed, revised and voted on by the entire group. The systematic literature search revealed 115,647 articles. We included 208 studies. We found strong evidence for treatment of people with ICH on organised stroke units, and secondary prevention of stroke with blood pressure lowering. We found weak evidence for scores for predicting macrovascular causes underlying ICH; acute blood pressure lowering; open surgery via craniotomy for supratentorial ICH; minimally invasive surgery for supratentorial ICH; decompressive surgery for deep supratentorial ICH; evacuation of cerebellar ICH > 15 mL; external ventricular drainage with intraventricular thrombolysis for intraventricular extension; minimally invasive surgical evacuation of intraventricular blood; intermittent pneumatic compression to prevent proximal deep vein thrombosis; antiplatelet therapy for a licensed indication for secondary prevention; and applying a care bundle. We found strong evidence against anti-inflammatory drug use outside of clinical trials. We found weak evidence against routine use of rFVIIa, platelet transfusions for antiplatelet-associated ICH, general policies that limit treatment within 24 h of ICH onset, temperature and glucose management as single measures (outside of care bundles), prophylactic anti-seizures medicines, and prophylactic use of temperature-lowering measures, prokinetic anti-emetics, and/or antibiotics. New evidence about the management of ICH has emerged since 2014, enabling this update of the ESO guideline to provide new recommendations and consensus statements. Although we made strong recommendations for and against a few interventions, we were only able to make weak recommendations for and against many others, or produce consensus statements where the evidence was insufficient to guide clinical decisions. Although progress has been made, many interventions still require definitive, high-quality evidence, underpinning the need for embedding clinical trials in routine clinical practice for ICH.
Keywords: Guideline; intracerebral haemorrhage; stroke; systematic review.
Plain language summary
BackgroundEvery year, around 3.4 million people have a type of stroke caused by bleeding in the brain that is not due to injury or another medical condition. The main causes of this kind of stroke include getting older, health issues like high blood pressure, and being exposed to air pollution. However, doctors and researchers are learning more and more about how to treat and prevent this condition, helping patients recover better and reducing the chances of it happening again. This guideline is an update of the last European Stroke Organisation guideline for people with bleeding in the brain, published in 2014.How We Created This GuideTo make sure this guide is based on the best available evidence, we followed a structured process recommended by the European Stroke Organisation (ESO) and the European Association of Neurosurgical Societies (EANS). We focused on finding the highest quality evidence about what care works best for patients with bleeding in the brain, and made recommendations guided by a framework called GRADE.We started with 37 important questions about care for people with stroke due to bleeding in the brain. To answer these, we looked at thousands of research papers and focused on the best available studies, especially ones where a treatment was compared reliably with an alternative. If there was not enough strong evidence to form a recommendation for clinical practice, we used expert opinions to create a consensus about a statement to guide clinical practice.What We FoundAfter looking at 115,647 studies, our findings for people with bleeding in the brain were:• What works best: We found strong evidence that patients get better when treated in specialized stroke units, and when their blood pressure is reduced to prevent more strokes.• What might help: There is weaker evidence supporting certain treatments, such as using scores to predict the cause of bleeding, early lowering of blood pressure, early use of some drugs to promote blood clotting, surgery to remove the bleeding (including approaches that use only a small hole in the skull), surgery to decompress the skull, drainage of blood in the fluid around the brain with a clot-busting drug, prevention of clots in veins by compression devices, and restarting blood-thinning medications for those who need them. There is also weaker evidence for patients getting better when a care bundle is used. These types of care require further study.• What should be avoided: We found strong evidence that anti-inflammatory drugs should not be used unless it’s part of a research study.• What might not help: We found weaker evidence against routine policies to limit treatment, controlling body temperature, controlling blood sugar, and routine treatment to prevent seizures, as well as evidence against giving a platelet transfusion (a type of blood product).• Uncertain areas: We did not find enough reliable evidence about tests to look for causes of bleeding, scores to predict outcome, early use of several drugs to promote blood clotting, surgery with drainage of fluid with a clot-busting drug, drainage of blood in the fluid around the brain, brain pressure monitoring, blood thinning drugs to prevent clots in veins, routine use of medicine to prevent seizures, blood thinning drugs and devices to prevent strokes and heart attacks for people with an irregular heartbeat, and statins to prevent strokes and heart attacks. In these cases, we provide expert opinions to help guide medical decisions and encourage more reliable research to be done.Why This MattersThis guideline summarises the best available evidence and expert opinions, to inform the care of people with stroke due to bleeding in the brain. This guideline may help doctors and other healthcare professionals to improve care for people with bleeding in the brain. Although a lot of progress has been made since the last edition of this guideline, more large, reliable, definitive clinical trials are needed to identify ways of improving outcome after bleeding in the brain.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Intellectual and financial disclosures of the module working group members are presented in Supplemental Table.
Figures

















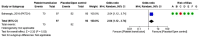




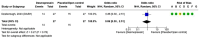
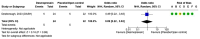



















































References
-
- Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–855. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

