Gut opportunistic pathogens contribute to high-altitude pulmonary edema by elevating lysophosphatidylcholines and inducing inflammation
- PMID: 40401968
- PMCID: PMC12210943
- DOI: 10.1128/spectrum.03057-24
Gut opportunistic pathogens contribute to high-altitude pulmonary edema by elevating lysophosphatidylcholines and inducing inflammation
Abstract
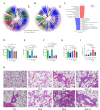
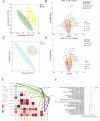
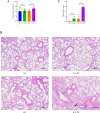
Gut microbiota have been found to promote hypoxia-induced intestinal injury. However, the role of gut microbiota in high-altitude pulmonary edema (HAPE), the preventive effect of synbiotic on HAPE, and the mechanisms by which they might work remain unknown. In this study, we aimed to investigate the role of gut microbiota in the pathogenesis of HAPE and to explore the underlying mechanisms involved. We performed a fecal microbiome analysis and found a significant decrease in intestinal Klebsiella and Escherichia-Shigella, along with a notable increase in intestinal Bifidobacterium and Lactobacillus, as volunteers recovered from acute mountain sickness (AMS). Gavage colonization with Klebsiella pneumoniae and Escherichia coli induced plasma inflammation, increased plasma lysophosphatidylcholine (LPC) levels, and contributed to HAPE in rats at a simulated altitude of 6,500 m. Conversely, a synbiotic containing Bifidobacterium, Lactiplantibacillus, fructooligosaccharides, and isomaltose-oligosaccharides significantly reduced the severity of HAPE. Cellular experiments and molecular dynamics simulations revealed that LPCs can cause damage and permeability to human pulmonary microvascular endothelial cell (HPMEC) and human pulmonary alveolar epithelial cell (HPAEpiC) monolayers under hypoxic conditions by disrupting cell membrane integrity. In addition, tail vein injection of LPCs promoted HAPE in mice at a simulated altitude of 6,500 m. In conclusion, this study describes a gut microbiota-LPCs/inflammation-HAPE axis, an important new insight into HAPE that will help open avenues for prevention and treatment approaches.
Importance: The role of the gut microbiota in high-altitude pulmonary edema (HAPE) is currently unknown. This study found that intestinal Klebsiella pneumoniae and Escherichia coli contribute to HAPE by inducing inflammation and increasing lysophosphatidylcholine (LPC) levels under hypoxic conditions. Conversely, a synbiotic containing Bifidobacterium, Lactiplantibacillus, fructooligosaccharides, and isomaltose-oligosaccharides significantly reduced the severity of HAPE. Further investigation revealed that LPCs can cause damage and permeability to human pulmonary microvascular endothelial cell (HPMEC) and human pulmonary alveolar epithelial cell (HPAEpiC) monolayers under hypoxic conditions by disrupting cell membrane integrity. These findings contribute to the understanding of the pathogenesis of HAPE and will benefit populations living at high altitude or traveling from low to high altitude.
Keywords: gut microbiota; high-altitude pulmonary edema; inflammation; lysophosphatidylcholines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Interventions for preventing high altitude illness: Part 2. Less commonly-used drugs.Cochrane Database Syst Rev. 2018 Mar 12;3(3):CD012983. doi: 10.1002/14651858.CD012983. Cochrane Database Syst Rev. 2018. PMID: 29529715 Free PMC article.
-
Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs.Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD009761. doi: 10.1002/14651858.CD009761.pub2. Cochrane Database Syst Rev. 2017. PMID: 28653390 Free PMC article.
-
Gut microbiota-targeted dietary supplementation with fermentable fibers and polyphenols prevents hypobaric hypoxia-induced increases in intestinal permeability.Am J Physiol Regul Integr Comp Physiol. 2025 Sep 1;329(3):R378-R399. doi: 10.1152/ajpregu.00109.2025. Epub 2025 Jul 23. Am J Physiol Regul Integr Comp Physiol. 2025. PMID: 40701649 Clinical Trial.
-
Interventions for treating acute high altitude illness.Cochrane Database Syst Rev. 2018 Jun 30;6(6):CD009567. doi: 10.1002/14651858.CD009567.pub2. Cochrane Database Syst Rev. 2018. PMID: 29959871 Free PMC article.
-
Multi-omics analyses reveal that hesperidin ameliorates high-altitude pulmonary hypertension by restoring gut-lung axis homeostasis.Phytomedicine. 2025 Sep;145:157069. doi: 10.1016/j.phymed.2025.157069. Epub 2025 Jul 11. Phytomedicine. 2025. PMID: 40680331
References
-
- Luks AM, Beidleman BA, Freer L, Grissom CK, Keyes LE, McIntosh SE, Rodway GW, Schoene RB, Zafren K, Hackett PH. 2024. Wilderness medical society clinical practice guidelines for the prevention, diagnosis, and treatment of acute altitude illness: 2024 update. Wilderness Environ Med 35:2S–19S. doi: 10.1016/j.wem.2023.05.013 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

