Noninvasive Evaluation of Prolonged-Release Pirfenidone in Compensated Liver Cirrhosis. ODISEA Study, a Randomised Trial
- PMID: 40402087
- PMCID: PMC12097196
- DOI: 10.1111/liv.70131
Noninvasive Evaluation of Prolonged-Release Pirfenidone in Compensated Liver Cirrhosis. ODISEA Study, a Randomised Trial
Abstract
Background: Advanced liver fibrosis (ALF) predicts an adverse prognosis in chronic liver disease. In addition to etiological treatment, a new approach to stop or reverse residual fibrosis is desirable.
Objective: To assess the efficacy and safety of prolonged-release pirfenidone (PR-PFD) versus placebo in compensated cirrhosis.
Methods: 180 patients with ALF (F4) were randomly assigned to: placebo, 1200 mg/d, and 1800 mg/d PR-PFD, plus standardised care, for 24mo. Frequency of lab tests: (3mo), liver stiffness measurement (LSM), FibroTest, ultrasound (US) (6mo), and endoscopy (annually).
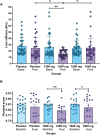
Results: Fibrosis evolution estimated from LSM was significantly lower only in the 1200 compared to placebo and 1800 groups (24.2 ± 2.4 vs. 15.4 ± 2.4; 27.6 ± 2.4 vs. 24.6 ± 2.4; 24.4 ± 2.3 vs. 23.3 ± 2.3 kPa, respectively, p < 0.001), in intergroup analysis, meeting the primary endpoint. Fibrotest was significantly lower only in the 1200 mg/d group, compared to baseline values (0.86 ± 0.02 vs. 0.83 ± 0.02 units, p < 0.001). Liver function test (LFT's) also improved as well as Model for End-Stage Liver Disease (MELD) score and quality of life (QoL). Decompensations occurred in 19 patients: 12 ascites (more frequent in placebo, p = 0.003), 5 variceal bleeding, 4 encephalopathies, 4 hepatocarcinomas. Adverse events were mainly mild gastrointestinal (n = 35, 48 and 46, p = 0.010) and cutaneous (n = 12, 15, and 22, p = 0.0001) in placebo, 1200 and 1800 mg/day, respectively.
Conclusion: PR-PFD at a dose of 1200 mg significantly decreased non-invasive liver fibrosis markers at 24 months and induced improvement in LFT's, MELD, and QoL in compensated cirrhosis, without safety concerns.
Trial registration: ClinicalTrials.gov identifier: NCT01046474.
Keywords: cirrhosis; fibrosis; prolonged‐release pirfenidone.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- GBD 2017 Cirrhosis Collaborators , “The Global, Regional, and National Burden of Cirrhosis by Cause in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017,” Lancet Gastroenterology & Hepatology 5, no. 3 (2020): 245–266, 10.1016/S2468-1253(19)30349-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

