Real-world observational study of infections in people treated with ocrelizumab for multiple sclerosis
- PMID: 40402264
- PMCID: PMC12098501
- DOI: 10.1007/s00415-025-13133-w
Real-world observational study of infections in people treated with ocrelizumab for multiple sclerosis
Abstract
Background: Anti-CD20 monoclonal antibodies are now a common first-line treatment for multiple sclerosis (MS). Rituximab, ocrelizumab and ofatumumab have all been associated with a dose-dependent risk of hypogammaglobulinaemia, but its relevance in clinical practice remains uncertain.
Objectives: To study infection rates over time in a real-world cohort of people treated with ocrelizumab for MS, and their relationship to serum immunoglobulin.
Design: Observational study of 152 people receiving ocrelizumab for MS followed for up to 5.6 years (mean 2.7 years).
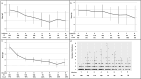
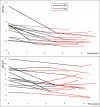
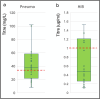
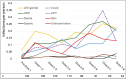
Results: Mean (SD) annualized changes in immunoglobulins during ocrelizumab treatment were IgM - 0.22 g/L/year (0.4), IgG - 0.38 g/L/year (0.9), IgA - 0.03 g/L/year. Rates of self-reported infection increased significantly during the first 4 years of treatment. Infection rates were not only associated with total immunoglobulin levels but also independently associated with age, comorbidity and female sex. We demonstrated for the first time that 29 out of 34 (87%) people on ocrelizumab with IgG in the lower normal range had sub-protective antibody responses to pneumococcus / haemophilus influenzae.
Conclusions: Real-world observational studies complement open label extensions of clinical trials, often by having a more representative cohort and more complete follow-up. Our data suggest that while serious infections are rare in people on ocrelizumab, non-serious infections become increasingly burdensome. We offer practical suggestions on mitigating the risk of infection on ocrelizumab and other anti-CD20 medications.
Keywords: Disease modifying therapy; Hypogammaglobulinaemia; Multiple sclerosis; Ocrelizumab; Treatment complications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: In the last 5 years ET has received honorarium for consulting work from Biogen, Janssen, Merck, Novartis, and Roche. She has received travel grants to attend or speak at educational meetings from Biogen, Merck, Neuroax, Roche, and Novartis. SJ has received support for conferences, speaker, advisory boards, trials, data and safety monitoring boards, studies and projects with CSL Behring, Takeda, Octapharma, Grifols, BPL, LFB, Kedrion, Pharming, Biocryst, Capitainer, Swedish Orphan Biovitrum, Biotest, Binding Site, GSK, Sanofi, UCB Pharma and HCRW. LD, RS, WJW report no conflicts of interest. Ethical statement: This human study has been approved by a UK research ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Figures
References
-
- Claflin SB, Tan B, Taylor BV (2019) The long-term effects of disease modifying therapies on disability in people living with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 10.1016/j.msard.2019.08.016 - PubMed
-
- Hauser SL et al (2020) Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 383(6):546–557. 10.1056/NEJMoa1917246 - PubMed
-
- Montalban X et al (2017) Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 376(3):209–220. 10.1056/NEJMoa1606468 - PubMed
-
- Hauser SL et al (2016) Efficacy and safety of ocrelizumab in relapsing multiple sclerosis: results of the IFN-beta-1a-controlled, double-blind, Phase III OPERA I and II studies. Mult Scler J 22(1):17–18
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

