Diagnostic value of genetic and epigenetic biomarker panels for colorectal cancer detection: a systematic review
- PMID: 40402271
- PMCID: PMC12098509
- DOI: 10.1007/s00384-025-04904-y
Diagnostic value of genetic and epigenetic biomarker panels for colorectal cancer detection: a systematic review
Abstract
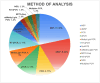
Purpose: Exploration of effective screening methods is imperative to improve current screening for colorectal cancer (CRC). Our aim was to systematically search the literature to identify and assess the diagnostic accuracy of both genetic and epigenetic biomarker panels for CRC detection using liquid biopsies for circulating tumour DNA (ctDNA) from stool, blood, or urine.
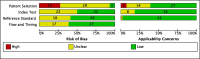
Methods: A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) with searches in Medline, Embase, CENTRAL, and Web Of Science from inception up to March 20, 2025, using pre-defined keywords. Study quality assessment was performed using QUADAS-2 tool (Quality Assessment for Diagnostic Accuracy Studies 2). Primary and secondary outcomes were panel performance (sensitivity and specificity) for CRC, advanced precancerous lesions (APL), and staging of disease.
Results: Forty-four studies were included. Exceptional performance for both CRC (sensitivity and specificity) and APL (sensitivity) was displayed by biomarker panels including methylated SDC2 with methylated SFRP1/2 (CRC: 91.5%/97.3%, APL: 89.2%) or methylated TFPI2 (CRC: 94.9%/98.1%, APL: 100%), and a 5-biomarker panel of mutational targets APC, Bat-26, KRAS, L-DNA, and p53 (CRC: 91.0%/93.0%, APL: 82.0%). Suboptimal APL sensitivities up to 57.0% were exhibited by Cologuard and variant panels (including KRAS, methylated BMP3, methylated NDRG4, FIT), and 47.8% for combinations including methylated SEPT9.
Conclusions: High-performance, candidate ctDNA biomarker panels with exceptional diagnostic accuracy for both CRC and APL have been identified. Further work should focus on the development of large-scale studies to justify their clinical implementation.
Keywords: Biomarker panels; Colorectal cancer; CtDNA; Detection; Epigenetic; Genetic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Bray F, Soerjomataram I (2015) The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. Disease Control Priorities, 3rd Edition vol 3, Cancer, pp 23–44. 10.1596/978-1-4648-0349-9_CH2 - PubMed
-
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN et al (2010) Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116:544–573. 10.1002/CNCR.24760 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

