Patient Number and Treatment Patterns in Cytomegalovirus Viremia and Disease Following Solid Organ and Hematopoietic Stem Cell Transplantation in Germany: Results of a Delphi Consensus Study
- PMID: 40402379
- PMCID: PMC12182504
- DOI: 10.1007/s12325-025-03210-x
Patient Number and Treatment Patterns in Cytomegalovirus Viremia and Disease Following Solid Organ and Hematopoietic Stem Cell Transplantation in Germany: Results of a Delphi Consensus Study
Abstract
Introduction: Management of cytomegalovirus (CMV) viremia/disease in transplant recipients may be complicated by toxicities and resistance to conventional antivirals, adding to the overall healthcare burden. Despite advances in analyzing real-world data in current years, little is known about refractory and resistant CMV. This study therefore aimed to characterize treatment patterns and patient numbers with special focus on refractory and resistant CMV.
Methods: Two classical three-round Delphi consensus panels with German clinical experts in CMV following solid organ transplantations (SOT) and hematopoietic stem cell transplantations (HSCT) were held between October and December 2021 using online questionnaires. Consensus was defined as agreement of 75% of participants.
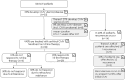
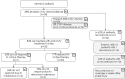
Results: Following SOT, experts agreed that on average 65% of SOT patients are not affected by CMV at all, while 35% of patients experience CMV viremia or disease. Of SOT patients treated with antiviral therapies, experts agreed that 90% respond to their first-line treatment and 10% do not. For HSCT, experts agreed that 62% of patients experience no CMV, while 38% of patients are diagnosed with either CMV viremia or CMV disease. It was further estimated that 23% HSCT patients receiving antiviral treatment do not respond to their first-line CMV treatment. Experts reached consensus on the reasons for non-response, suggesting that among non-responders, 55% were intolerant, while 45% of non-responders were refractory/resistant to first-line treatment.
Conclusion: Based on the current incidence of transplantations in Germany, experts estimated that 103 SOT and 225 HSCT patients need second-line CMV treatment annually.
Keywords: Cytomegalovirus; Hematopoietic stem-cell transplantation; Refractory and resistant cytomegalovirus; Solid organ transplantation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Daniel Teschner has received research grants from Gilead, honoraria from and served as a consultant to AbbVie, BioNTech, Gilead, iQone, Jazz, Mikrogen, MSD, Noscendo, Octapharma, Pfizer, Tillotts, and Takeda, honoraria from AstraZeneca, F2G, Janssen, Novartis, and Sanofi, and travel grants from AbbVie, Astellas, Celgene, Gilead, Jazz, Medac, MSD, and Tillotts. Jana Knop and Christian Piehl are employees of Takeda Pharma Vertrieb GmbH & Co. KG. Christian Piehl holds Takeda stocks. Tino Schubert is owner and employee of LinkCare GmbH, which received consulting honoraria from Takeda Pharma Vertrieb GmbH and Mallinckrodt Pharmaceuticals. Oliver Witzke received research grants for clinical studies, speaker’s fees, honoraria, and travel expenses from Alexion, Amgen, Astellas, Basilea, Biotest, Bristol Myers Squibb, Chiesi, Correvio, Gilead, Hexal, Janssen, Dr. F. Köhler Chemie, MSD, Novartis, Pfizer, Roche, Sanofi, Takeda, Teva, and UCB, as well as an unrestricted grant from the Rudolf-Ackermann-Stiftung (Stiftung für Klinische Infektiologie). Ethical Approval: Participants were fully informed about the study’s objectives and the intent to publish the results. Consent was given voluntarily through signed contracts. Participant answers were anonymized, and no communication occurred between them during the study. Data protection adhered to GDPR regulations, with analyses conducted pseudonymously to ensure privacy. Ethical committee consultation was unnecessary as the study only involved physician responses, with no patient information and no interference with personal integrity.
Figures


References
-
- Avery RK, Alain S, Alexander BD, Blumberg EA, Chemaly RF, Cordonnier C, Duarte RF, Florescu DF, Kamar N, Kumar D, Maertens J, Marty FM, Papanicolaou GA, Silveira FP, Witzke O, Wu J, Sundberg AK, Fournier M, SOLSTICE Trial Investigators. Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial. Clin Infect Dis. 2022;75(4):690–701. - PMC - PubMed
-
- Baden LR, Swaminathan S, Angarone M, Blouin G, Camins BC, Casper C, Cooper B, Dubberke ER, Engemann AM, Freifeld AG, Greene JN, Ito JI, Kaul DR, Lustberg ME, Montoya JG, Rolston K, Satyanarayana G, Segal B, Seo SK, Shoham S, Taplitz R, Topal J, Wilson JW, Hoffmann KG, Smith C. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882–913. - PubMed
-
- Boivin G, Goyette N, Rollag H, Jardine AG, Pescovitz MD, Asberg A, Ives J, Hartmann A, Humar A. Cytomegalovirus resistance in solid organ transplant recipients treated with intravenous ganciclovir or oral valganciclovir. Antivir Ther. 2009;14(5):697–704. - PubMed
-
- Charlotte R, François P, Jonathan M, Véronique B, Olivier B, Tristan D, Séverine F, Jérôme L, Adrien T, Claire D, Espérie B, Eve C, Antoine R. Use of anti-CMV immunoglobulins in lung transplant recipients: the French experience. Transpl Infect Dis. 2021;23(6): e13754. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

