Metabolomic Profiling of Aqueous Humor From Glaucoma Patients Identifies Metabolites With Anti-Inflammatory and Neuroprotective Potential in Mice
- PMID: 40402521
- PMCID: PMC12110543
- DOI: 10.1167/iovs.66.5.28
Metabolomic Profiling of Aqueous Humor From Glaucoma Patients Identifies Metabolites With Anti-Inflammatory and Neuroprotective Potential in Mice
Abstract
Purpose: Metabolomic profiling of aqueous humor from primary open-angle glaucoma (POAG) patients using targeted metabolomics analysis and assessment of the potential anti-neuroinflammatory and neuroprotective roles of key dysregulated metabolites in a mouse model of retinal neuroinflammation.
Methods: A targeted metabolomics was performed on aqueous humor from POAG patients (n = 19) and healthy subjects (n = 10) via LC-MS/MS. In vitro neuroprotection studies were performed using a mouse cone photoreceptor cell line (661W) exposed to oxidative stress. For in vivo therapeutic studies, a few key dysregulated metabolites were delivered either topically via extracellular vesicle (EV)-mediated delivery or intravitreally into a C57BL/6 mouse model of retinal neuroinflammation. The neuroprotective and anti-neuroinflammatory properties were determined in the presence and absence of metabolites through pattern electroretinography, TUNEL, and quantitative PCR analyses.
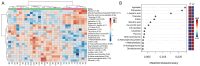
Results: Among the 135 endogenous metabolites identified, 31 metabolites showed significant dysregulation in POAG. Metabolite set enrichment analysis revealed that these altered metabolites were associated with dysregulation of multiple key cellular pathways, including glycolysis, pentose phosphate pathway, short-/long-chain fatty acid metabolism, mitochondrial β-oxidation, and electron transport chain under glaucomatous conditions. Among these differentially expressed metabolites, a putative neuromodulator (agmatine) and a vitamin (thiamine) significantly decreased in POAG patients. Intravitreal or EV-mediated topical delivery of agmatine and thiamine significantly reduced the inflammatory response and protected retinal ganglion cell function against neuroinflammatory damage in the mouse retina. Agmatine and thiamine treatment also significantly protected photoreceptor cells from oxidative stress-induced cell death and attenuated the inflammatory cytokine response.
Conclusions: Our results revealed significant metabolic alterations in POAG that affect key cellular functions. Agmatine and thiamine could be potential immunomodulatory or neuroprotective drugs to treat or prevent neuroinflammatory damage to the retina during glaucoma.
Conflict of interest statement
Disclosure:
Figures






References
-
- Jayaram H, Kolko M, Friedman DS, Gazzard G.. Glaucoma: now and beyond. Lancet. 2023; 402: 1788–1801. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

