Wag31, a membrane tether, is crucial for lipid homeostasis in mycobacteria
- PMID: 40402572
- PMCID: PMC12097788
- DOI: 10.7554/eLife.104268
Wag31, a membrane tether, is crucial for lipid homeostasis in mycobacteria
Abstract
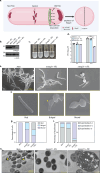
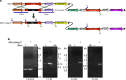
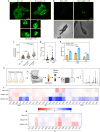
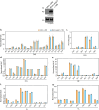
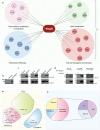
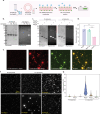
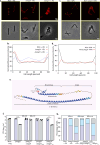
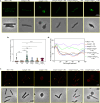
The mycobacterial cytoskeletal protein Wag31 is necessary for maintaining cell shape and directing cellular growth and elongation. Wag31 has a characteristic N-terminal DivIVA-domain and a C-terminal coiled-coil domain. While the role of Wag31 in polar elongation is known, there is limited mechanistic insight on how it orchestrates growth and elongation. In this report, we delineate roles of the N- and C-terminal domains of Wag31 using genetics, state-of-the-art multi-omics, biochemical, and imaging approaches. We show that Wag31 predominantly interacts with several membrane-associated proteins involved in lipid metabolism, cell wall synthesis, and division. Native levels of Wag31 are critical for the maintenance and distribution of membrane lipids. Both depletion and overexpression of Wag31 perturb lipid homeostasis, leading to the formation of intracellular lipid inclusions (ILIs). Protein-lipid crosslinking and imaging studies reveal that purified Wag31 can bind and effectively tether cardiolipin (CL)-containing liposomes. We further show that the tethering activity lies in the DivIVA-domain containing N-terminal of Wag31 while the C-terminal mediates protein-protein interactions of Wag31. Despite retaining its ability to interact with partner proteins, the DivIVA-domain-deleted Wag31 mutant shows defects in liposome tethering in vitro and non-polar localization of CL in vivo, which eventually causes lethality. Our study suggests that membrane tethering 'licenses' Wag31 to form scaffolds that help orchestrate protein-lipid and protein-protein interactions necessary for mycobacterial growth and survival.
Keywords: Mycobacterium smegmatis; Wag31; infectious disease; intracellular lipid inclusions; lipid homeostasis; membrane tether; microbiology.
© 2025, Kapoor et al.
Conflict of interest statement
YK, HK, DD, AC, AP, AS, SK, ND, TP No competing interests declared, VN Reviewing editor, eLife
Figures



Update of
- doi: 10.1101/2024.10.22.619650
- doi: 10.7554/eLife.104268.1
- doi: 10.7554/eLife.104268.2
References
-
- Carel C, Nukdee K, Cantaloube S, Bonne M, Diagne CT, Laval F, Daffé M, Zerbib D. Mycobacterium tuberculosis proteins involved in mycolic acid synthesis and transport localize dynamically to the old growing pole and septum. PLOS ONE. 2014;9:e97148. doi: 10.1371/journal.pone.0097148. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

