T-follicular helper cell profiles differ by malaria antigen and for children compared to adults
- PMID: 40402846
- PMCID: PMC12097790
- DOI: 10.7554/eLife.98462
T-follicular helper cell profiles differ by malaria antigen and for children compared to adults
Abstract
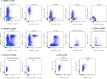
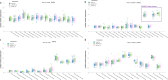
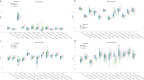
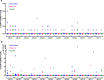
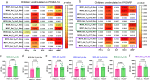
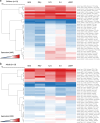
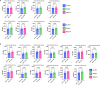
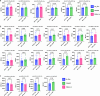
Circulating T-follicular helper (cTFH) cells have the potential to provide an additional correlate of protection against Plasmodium falciparum (Pf) as they are essential to promote B-cell production of long-lasting antibodies. Assessing the specificity of cTFH subsets to individual malaria antigens is vital to understanding the variation observed in antibody responses and identifying promising malaria vaccine candidates. Using spectral flow cytometry and unbiased clustering analysis, we assessed antigen-specific cTFH cell recall responses in vitro to malaria vaccine candidates Pf-schizont egress antigen-1 (PfSEA-1A) and Pf-glutamic acid-rich protein (PfGARP) within a cross-section of children and adults living in a malaria-holoendemic region of western Kenya. In children, a broad array of cTFH subsets (defined by cytokine and transcription factor expression) were reactive to both malaria antigens, PfSEA-1A and PfGARP, while adults had a narrow profile centering on cTFH17- and cTFH1/17-like subsets following stimulation with PfGARP only. Because TFH17 cells are involved in the maintenance of memory antibody responses within the context of parasitic infections, our results suggest that PfGARP might generate longer-lived antibody responses compared to PfSEA-1A. These findings have intriguing implications for evaluating malaria vaccine candidates as they highlight the importance of including cTFH profiles when assessing interdependent correlates of protective immunity.
Keywords: Kenya; P. falciparum; T-follicular helper; immunology; inflammation; pf-malaria; vaccine candidates.
© 2024, Forconi et al.
Conflict of interest statement
CF, CN, HW, BO, SP, JO, AM No competing interests declared, JK Principal investigator on 1R01AI127699-01A1, which supported this study; holds several patents related to the use of PfSEA-1 and PfGARP as vaccine candidates for P. falciparum and has consulted for and is an equity holder in Ocean Biomedical
Figures



















Update of
-
T follicular helper cell profiles differ by malaria antigen and for children compared to adults.bioRxiv [Preprint]. 2025 Feb 11:2024.04.13.589352. doi: 10.1101/2024.04.13.589352. bioRxiv. 2025. Update in: Elife. 2025 May 22;13:RP98462. doi: 10.7554/eLife.98462. PMID: 38659768 Free PMC article. Updated. Preprint.
References
-
- Aydemir O, Janko M, Hathaway NJ, Verity R, Mwandagalirwa MK, Tshefu AK, Tessema SK, Marsh PW, Tran A, Reimonn T, Ghani AC, Ghansah A, Juliano JJ, Greenhouse BR, Emch M, Meshnick SR, Bailey JA. Drug-resistance and population structure of Plasmodium falciparum across the democratic republic of congo using high-throughput molecular inversion probes. The Journal of Infectious Diseases. 2018;218:946–955. doi: 10.1093/infdis/jiy223. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

