Exploring Long Arm Amide-Linked Side Chains in the Design of Antifungal Azole Inhibitors of Sterol 14α-Demethylase (CYP51)
- PMID: 40403151
- PMCID: PMC12169614
- DOI: 10.1021/acs.jmedchem.4c02922
Exploring Long Arm Amide-Linked Side Chains in the Design of Antifungal Azole Inhibitors of Sterol 14α-Demethylase (CYP51)
Abstract
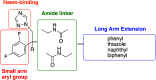
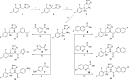
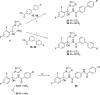
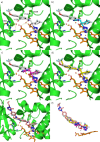
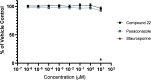
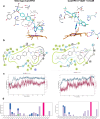
The rise in fungal drug resistance has exacerbated the treatment of invasive fungal infections, most commonly caused by Candida. This research describes the synthesis of extended "long-arm" azole antifungals that were evaluated against wild-type and resistant fungal species. Biphenyl derivative 22 was the most effective derivative, displaying potent inhibitory activity against Saccharomyces, Candida, and Cryptococcus CYP51 enzymes, including in resistant strains, in comparison with posaconazole. The X-ray crystal structure of S-22 complexed with S. cerevisiae CYP51 showed a hydrogen bond between the oxygen of the trifluoromethoxy group of S-22 and the His381 side chain of S. cerevisiae CYP51, which is postulated to contribute significantly to its binding, and stabilization in the presence of the S. cerevisiae CYP51 Y140F/H, C. parapsilosis and C. auris CYP51 Y132F mutations and the C. auris K143R mutation. Computational studies and IC50 evaluation of compound 22 vs C. albicans wild-type, Y132F, and Y132H/K143 mutant strains supported MIC observations.
Figures

References
-
- WHO fungal priority pathogens list to guide research, development and public health action; World Health organization: Geneva, 2022. Available from: https://www.who.int/publications/i/item/9789240060241 (Accessed 4 October 2024).
-
- Sati H., Alastruey-Izquierdo A., Perfect J., Govender N. P., Harrison T. S., Chiller T., Sorrell T. C., Bongomin F., Oladele R., Chakrabati A., Wahyuningsih R., Colombo A. L., Rodriguez-Tudela J. L., Beyrer C., Ford N.. HIV and fungal priority pathogens. Lancet. 2023;10:e750–e754. doi: 10.1016/S2352-3018(23)00174-1. - DOI - PMC - PubMed
-
- Dewi I. M. W., Fauziah N., Ekawardhani S., Andriyoko B., Adawiyah R., Hartantri Y., Soeroto A. Y., Alisjahbana B., Denning D. W., Wahyuningsih R.. Antibodies against Histoplasma capsulatum and Aspergillus fumigatus among chronic TB patients in Indonesia: a cross-sectional study. Med. Mycol. 2023;61:myad036. doi: 10.1093/mmy/myad036. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

