Cyanoglobule lipid droplets are a stress-responsive metabolic compartment of cyanobacteria and the progenitor of plant plastoglobules
- PMID: 40403160
- PMCID: PMC12223511
- DOI: 10.1093/plcell/koaf127
Cyanoglobule lipid droplets are a stress-responsive metabolic compartment of cyanobacteria and the progenitor of plant plastoglobules
Abstract
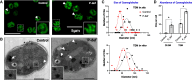
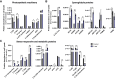
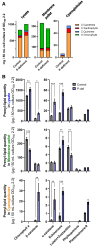
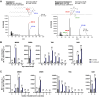
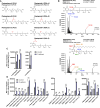
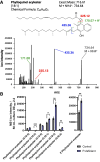
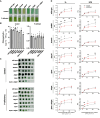
Lipid droplets (LDs) are well integrated into multiple facets of cellular physiology and potentially represent an effective platform for engineering heterologous metabolic pathways. LDs of chloroplasts, known as plastoglobules, mediate stress tolerance through dynamic and reversible changes to morphology and molecular composition. However, the dynamics and functional role(s) of cyanobacterial LDs are almost wholly unknown. Here, we have characterized the morphological behavior and quantitative proteome and lipidome of cyanobacterial LDs of Synechocystis sp. PCC 6803 grown under permissive or phosphorous-deficient media for 7 d. Our results show that cyanobacterial LDs are a metabolically active subcompartment of cyanobacteria with dynamic morphology and composition. The cyanobacterial LD proteome and lipidome are qualitatively similar to those of plant plastoglobules including an enrichment of prenyl lipids and the presence of twelve orthologs of Arabidopsis thaliana plastoglobule proteins. In view of these results, we propose that cyanobacterial LDs be named as cyanoglobules. In addition, we established that various plastoquinone derivatives containing hydroxyl and/or acyl groups on their solanesyl tail or quinone head accumulate in cyanoglobules. Deletion mutants of selected cyanoglobule proteins exhibit impairments in growth, pigmentation, and photosynthesis. Our results collectively support an evolutionary relationship between cyanoglobules and plastoglobules and reveal a possible central role for cyanoglobules in organismal physiology and stress adaptation.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

