Intervention With WhatsApp Messaging to Compare the Effect of Self-Designed Messages and Standardized Messages in Adherence to Antiretroviral Treatment in Young People Living With HIV in a Hospital in Lima, Peru: Protocol for a Nonblinded Randomized Controlled Trial
- PMID: 40403302
- PMCID: PMC12141961
- DOI: 10.2196/66941
Intervention With WhatsApp Messaging to Compare the Effect of Self-Designed Messages and Standardized Messages in Adherence to Antiretroviral Treatment in Young People Living With HIV in a Hospital in Lima, Peru: Protocol for a Nonblinded Randomized Controlled Trial
Abstract
Background: Young people living with HIV face challenges in consistently adhering to antiretroviral therapy (ART). Although mobile health interventions, particularly those using SMS text messaging, have been implemented to improve ART adherence, many lack a focus on specific behavioral mechanisms. Interventions incorporating behavioral change techniques (BCTs), especially those emphasizing customization, may enhance effectiveness. WhatsApp offers potential for delivering tailored, behaviorally grounded interventions with diverse communication features. We hypothesize that WhatsApp messages self-designed by participants, with spontaneous targeting of BCTs, could be more effective than standard WhatsApp messages designed by the researchers to improve ART adherence.
Objective: The objective of this study is to evaluate the effectiveness of WhatsApp messages created by participants (self-designed) compared to WhatsApp messages designed by the researchers (standardized) over adherence to ART at 16 weeks of intervention in young people living with HIV who receive HIV care under routine conditions at a public hospital in Lima, Peru.
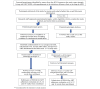
Methods: A 2-arm randomized controlled trial with a parallel assignment of 1:1, with no blinding of study intervention, was performed. Eligible participants are consenting people living with HIV aged 18-29 years who receive HIV care at the study center and whose mobile phones support WhatsApp. Following informed consent and a baseline survey on clinical and personal preferences (eg, timing and frequency of messages), participants are randomized to the control group (messages designed by the research team) or to the experimental group (messages designed by participants), stratified by sex, educational level, current ART intake, and history of ART abandonment. Participants in both groups receive up to 3 WhatsApp messages per week for 16 weeks. ART adherence, the primary outcome, is measured using the Simplified Medication Adherence Questionnaire (SMAQ) at 4, 8, 12, and 16 weeks. Monthly feedback questionnaires on user experience are also administered. The WhatsApp chat format allows two-way communication between participants and the research team throughout the study. We will compare ART adherence between 2 groups at 16 weeks under the intention-to-treat principle, with no interim analysis planned. Based on an estimated 10% difference in adherence, 78.9% power, and a 2-sided α of .05, the target sample size was set at 120, later increased to 131 to include a 2-week pilot phase.
Results: In March 2024, we started enrolling and randomizing participants. The study follow-up will continue until the last participant completes 16 weeks of intervention (November 2024). As of February 2025, we are in the process of data curation.
Conclusions: This trial will compare the effectiveness of standardized vs self-designed WhatsApp messages on ART adherence measured at 16 weeks among young people living with HIV receiving routine care in a low-resource setting in Lima.
Trial registration: ClinicalTrials.gov NCT06500013; https://clinicaltrials.gov/ct2/show/NCT06500013.
International registered report identifier (irrid): DERR1-10.2196/66941.
Keywords: ART; HIV; Peru; WhatsApp; adherence; antiretroviral treatment; behavioral change technique; design; digital app; evaluation; mHealth; messaging intervention; mobile phone messaging; people living with HIV; randomized controlled trial; self-design; young people.
©Jeffrey Freidenson-Bejar, Dianne Espinoza, Rodrigo Calderon-Flores, Fernando Mejia, Elsa González-Lagos. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 22.05.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Two-way mobile phone intervention compared with standard-of-care adherence support after second-line antiretroviral therapy failure: a multinational, randomised controlled trial.Lancet Digit Health. 2019 May;1(1):e26-e34. doi: 10.1016/S2589-7500(19)30006-8. Epub 2019 May 6. Lancet Digit Health. 2019. PMID: 31528850 Free PMC article. Clinical Trial.
-
Attitudes, Beliefs, and Willingness Toward the Use of mHealth Tools for Medication Adherence in the Florida mHealth Adherence Project for People Living With HIV (FL-mAPP): Pilot Questionnaire Study.JMIR Mhealth Uhealth. 2019 Jul 3;7(7):e12900. doi: 10.2196/12900. JMIR Mhealth Uhealth. 2019. PMID: 31271150 Free PMC article.
-
A Culturally Adapted SMS Text Messaging Intervention to Promote Antiretroviral Therapy Adherence Among African Americans: Protocol for a Single-Arm Trial.JMIR Res Protoc. 2020 Dec 10;9(12):e21592. doi: 10.2196/21592. JMIR Res Protoc. 2020. PMID: 33300885 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
A systematic review and meta-analysis in the effectiveness of mobile phone interventions used to improve adherence to antiretroviral therapy in HIV infection.BMC Public Health. 2019 Jul 9;19(1):915. doi: 10.1186/s12889-019-6899-6. BMC Public Health. 2019. PMID: 31288772 Free PMC article.
References
-
- Estimated HIV incidence and prevalence in the United States, 2018-2022. HIV Surveillance Supplemental Report. Centers for Disease Control and Prevention. 2024. May, [2025-04-16]. https://www.cdc.gov/hiv-data/nhss/estimated-hiv-incidence-and-prevalence... .
-
- Green D, Tordoff DM, Kharono B, Akullian A, Bershteyn A, Morrison M, Garnett G, Duerr A, Drain PK. Evidence of sociodemographic heterogeneity across the HIV treatment cascade and progress towards 90-90-90 in sub-Saharan Africa - a systematic review and meta-analysis. J Int AIDS Soc. 2020;23(3):e25470. doi: 10.1002/jia2.25470. https://europepmc.org/abstract/MED/32153117 - DOI - PMC - PubMed
-
- Pence B, Bengtson A, Boswell S, Christopoulos K, Crane H, Geng E, Keruly JC, Mathews WC, Mugavero MJ. Who will show? Predicting missed visits among patients in routine HIV primary care in the United States. AIDS Behav. 2019;23(2):418–426. doi: 10.1007/s10461-018-2215-1. https://europepmc.org/abstract/MED/30006790 10.1007/s10461-018-2215-1 - DOI - PMC - PubMed
-
- Mbuagbaw L, van der Kop M L, Lester R, Thirumurthy H, Pop-Eleches C, Ye C, Smieja M, Dolovich L, Mills EJ, Thabane L. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): an individual patient data meta-analysis of randomised trials. BMJ Open. 2013;3(12):e003950. doi: 10.1136/bmjopen-2013-003950. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=24345901 bmjopen-2013-003950 - DOI - PMC - PubMed
-
- Kanters S, Park J, Chan K, Socias M, Ford N, Forrest J, Thorlund K, Nachega JB, Mills EJ. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–e40. doi: 10.1016/S2352-3018(16)30206-5.S2352-3018(16)30206-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials