Impact of germline variants on breast and ovarian cancer risk in Japanese women: an original cohort study and meta-analysis
- PMID: 40403695
- PMCID: PMC12148598
- DOI: 10.1016/j.ebiom.2025.105758
Impact of germline variants on breast and ovarian cancer risk in Japanese women: an original cohort study and meta-analysis
Abstract
Background: Pathogenic variants (PVs) of BRCA1 and BRCA2 predispose individuals to a higher risk of breast and ovarian cancer; however, the precise risks posed by other cancer susceptibility genes remain unclear, particularly in Asian populations.
Methods: We executed a case-control study of 11 and 26 genes associated with breast and ovarian cancer susceptibility, respectively, in 7220 women with breast cancer, 2464 women with ovarian cancer, and 4032 controls from a multicentre, hospital-based registry in Japan. Furthermore, we conducted a meta-analysis of 23,193 patients with breast and/or ovarian cancer and 31,190 controls from six other hospital-based studies.
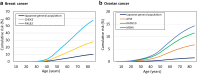
Findings: Overall, 395 (5.5%) patients with breast cancer and 331 (13.4%) patients with ovarian cancer harboured PVs. Meta-analyses revealed that PVs of BRCA1, BRCA2, CHEK2, PALB2, and TP53 were associated significantly with breast cancer risk (P < 0.001), while PVs of ATM, BRCA1, BRCA2, MSH6, and RAD51D were associated significantly with ovarian cancer risk (P < 0.001). PVs in the BRCA1 DNA-binding domain were associated with a younger age at diagnosis after adjusting for cancer type and family history (β = -3.79, 95% CI = -7.16 to -0.41; P = 0.028).
Interpretation: These results provide information about genes associated with breast and ovarian cancer risk in Asian women, as well as guidance for management of PV carriers.
Funding: The study was funded by AMED (JP15ck010609, 19cm0106605h0003, 23ama221520h0001, and JP19kk0305010), by a Health Labour Sciences Research Grant (202108001B), by JSPS KAKENHI (JP18K16292, 20H03668, 23H02955, 17H06162, 20H03695, and 16H06277), and by a Grant-in-Aid for the Genome Research Project from Yamanashi Prefecture.
Keywords: Breast cancer; Cancer predisposition genes; Cumulative cancer risk; Ovarian cancer.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Masayuki Yoshida reported receiving personal fees from Roche Japan, Agilent Technologies, Chugai Pharma, Ono Yakuhin, MSD, and Daiichi Sankyo. He has participated on a Data Safety Monitoring Board or Advisory Board for Daiichi Sankyo. Kenichi Harano reported receiving grants from AstraZeneca, Chugai, Daiichi Sankyo, MSD, and Takeda, and personal fees from AstraZeneca, Chugai, Eisai, MSD, Taiho, and Takeda. He has participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Chugai, Daiichi Sankyo, Taiho, and Takeda. Takashi Yamanaka reported receiving personal fees from Daiichi Sankyo, Eli Lilly Japan, AstraZeneca, Chugai, Pfizer Japan, Kyowa Kirin, and Taiho. Kazunoshin Tachibana reported receiving grants from Chugai, Eisai, Taiho, Takeda, MSD, Daiichi Sankyo, Eli Lilly, Asahi Kasei, Nihon Kayaku, Kyowa Kirin, Astellas, and Maruho, and personal fees from Chugai, AstraZeneca, Pfizer, Eisai, Daiichi Sankyo, Eli Lilly, MSD, Kyowa Kirin, Teijin, Taiho, PDR Pharma, and Exact Sciences. Chikako Shimizu reported receiving personal fees from Chugai and has participated on a Data Safety Monitoring Board or Advisory Board for Daiichi Sankyo. Akihiko Shimomura reported receiving grants from Chugai Pharmaceutical, AstraZeneca, and Eisai, and personal fees from AstraZeneca, Daiichi Sankyo, Pfizer, Eli Lilly, MSD, Chugai Pharmaceutical, Nihon Medi-Physics, Taiho Pharmaceutical, and Exact Sciences. Takahiro Kogawa reported receiving grants from Eli Lilly, AstraZeneca, and Guardant Health; consulting fees from Daiichi Sankyo and Astellas Pharma; and personal fees from Daiichi Sankyo, Ono Pharma, Gilead Sciences, Astellas Pharma, Eisai, AstraZeneca, Taiho Pharma, and Chugai Pharma. He has received payment for expert testimony from Astellas Pharma and support for attending meetings from Pfizer and Eisai. He has participated on a Data Safety Monitoring Board or Advisory Board for Daiichi Sankyo, Ono Pharma, Gilead Sciences, Oncotherapy Sciences, Eisai, AstraZeneca, and Taiho Pharma. Tohru Ohtake reported receiving grants from Chugai, Eisai, Taiho, Takeda, Asahi Kasei, Daiichi Sankyo, Eli Lilly, Nihon Kayaku, and Kyowa Kirin, and personal fees from Chugai, Pfizer, AstraZeneca, Eisai, Daiichi Sankyo, Eli Lilly, Kyowa Kirin, Novartis, FUJIFILM Toyama Chemical, Johnson & Johnson, Asahi Kasei, Exact Sciences, Otsuka, and MSD. Keiya Fujimori reported receiving grants from MSD, Terumo, Taiho, Chugai, Kaken, Daiichi Sankyo, Asuka, Mochida, and Bayer, and personal fees from Chugai, Asuka, AstraZeneca, Takeda, Mochida, Konica Minolta Japan, Tsumura, GE Health Japan, Zeria, Johnson & Johnson, Nobelpharma, Otsuka, MSD, Becton Dickinson, and Fuji Pharma. He also holds stock in Takeda. Aikou Okamoto reported receiving grants from Meiji Holdings, ASKA Pharmaceutical, Taiho Pharmaceutical, Chugai Pharmaceutical, Mochida Pharmaceutical, Eisai, Kaken Pharmaceutical, Fuji Pharma, Merck Biopharma Japan, MSD, Takeda Pharmaceutical, and AbbVie, and personal fees from MSD, Takeda Pharmaceutical, Bayer, Kaken Pharmaceutical, Chugai Pharmaceutical, Fuji Pharma, Zeria Pharmaceutical, ASKA Pharmaceutical, and AstraZeneca. Masao Omata reported receiving personal fees from On, Gilead Sciences, Janssen, Bayer, AbbVie, Chugai, AstraZeneca, FUJIFILM Wako Pure Chemical, and ASKA. Takafumi Watanabe reported receiving personal fees from Chugai, AstraZeneca, and MSD. Tatsuya Onishi reported receiving grants from Daiichi Sankyo and Bayer Pharma, and personal fees from Daiichi Sankyo. Toshinari Yamashita reported receiving grants from Chugai, Taiho, Nippon Kayaku, Eli Lilly, Daiichi Sankyo, Pfizer, AstraZeneca, Seagen, MSD, Kyowa Kirin, Ono, Gilead Sciences, and Eisai, and personal fees from Chugai, Eisai, Daiichi Sankyo, Taiho, Nippon Kayaku, AstraZeneca, Kyowa Kirin, Pfizer, Eli Lilly, Novartis Pharma, and MSD. Yoichi Naito reported receiving grants from AbbVie, Ono, Daiichi Sankyo, Taiho, Pfizer, Boehringer Ingelheim, Eli Lilly, Eisai, AstraZeneca, Chugai, and Bayer, and personal fees from AstraZeneca, Eisai, Ono, Guardant, Takeda, Eli Lilly, Novartis, Pfizer, Chugai, PDR Pharma, Nihon Kayaku, Taiho, Bristol Myers Squibb, Bayer, Daiichi Sankyo, and MSD. Kan Yonemori reported receiving grants from MSD, Daiichi Sankyo, Merck Biopharma, AstraZeneca, Taiho, Pfizer, Novartis, Takeda, Chugai, Ono, Sanofi, Seagen, Eisai, Eli Lilly, Genmab, Boehringer Ingelheim, Kyowa Hakko Kirin, Nihon Kayaku, and Haihe; personal fees from Pfizer, Eisai, AstraZeneca, Eli Lilly, Takeda, Chugai, Fuji Film Pharma, PDR Pharma, MSD, Boehringer Ingelheim, Ono, Daiichi Sankyo, Bayer, Janssen, Astellas, Bristol Myers Squibb, Novartis, and Sanofi; and has participated on a Data Safety Monitoring Board or Advisory Board for Eisai, AstraZeneca, Sanofi, Genmab, Gilead, OncXerna, Takeda, Novartis, MSD, and Henlius. All remaining authors declare no conflicts of interest.
Figures


References
-
- Daly M.B., Pal T., Berry M.P., et al. Genetic/Familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. - PubMed
-
- Cybulski C., Wokolorczyk D., Jakubowska A., et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29(28):3747–3752. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

