Enhancer AAV toolbox for accessing and perturbing striatal cell types and circuits
- PMID: 40403704
- PMCID: PMC12237622
- DOI: 10.1016/j.neuron.2025.04.035
Enhancer AAV toolbox for accessing and perturbing striatal cell types and circuits
Abstract
We present an enhancer-AAV toolbox for accessing and perturbing striatal cell types and circuits. Best-in-class vectors were curated for accessing major striatal neuron populations including medium spiny neurons (MSNs), direct- and indirect-pathway MSNs, Sst-Chodl, Pvalb-Pthlh, and cholinergic interneurons. Specificity was evaluated by multiple modes of molecular validation, by three different routes of virus delivery, and with diverse transgene cargos. Importantly, we provide detailed information necessary to achieve reliable cell-type-specific labeling under different experimental contexts. We demonstrate direct pathway circuit-selective optogenetic perturbation of behavior and multiplex labeling of striatal interneuron types for targeted analysis of cellular features. Lastly, we show conserved in vivo activity for exemplary MSN enhancers in rats and macaques. This collection of striatal enhancer AAVs offers greater versatility compared to available transgenic lines and can readily be applied for cell type and circuit studies in diverse mammalian species beyond the mouse model.
Keywords: Pvalb; Sst-Chodl; basal ganglia; caudoputamen; cholinergic; macaque; medium spiny neuron; spiny projection neuron; striatonigral; striatopallidal.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.P.L., B.B.G., J.K.M., and E.S.L. are founders and board members of EpiCure Therapeutics. J.T.T., B.P.L., E.S.L., T.L.D., B. Tasic, H.Z., and J.K.M. are co-inventors on patent application PCT/US2021/45995 Artificial expression constructs for selectively modulating gene expression in striatal neurons. J.T.T., B.P.L., T.L.D., B. Tasic, and T.E.B. are co-inventors on provisional patent application US 63/582,759 Artificial expression constructs for modulating gene expression in the basal ganglia. H.Z. is on the scientific advisory board of MapLight Therapeutics, Palo Alto, CA.
Figures


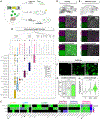




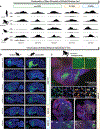
Update of
-
Enhancer AAV toolbox for accessing and perturbing striatal cell types and circuits.bioRxiv [Preprint]. 2025 Mar 20:2024.09.27.615553. doi: 10.1101/2024.09.27.615553. bioRxiv. 2025. Update in: Neuron. 2025 May 21;113(10):1507-1524.e17. doi: 10.1016/j.neuron.2025.04.035. PMID: 39386678 Free PMC article. Updated. Preprint.
References
-
- DeLong MR, and Wichmann T (2007). Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 64, 20–24. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

