Comparability, acceptability and longitudinal adherence with digital emPHasis-10 in pulmonary arterial hypertension
- PMID: 40404213
- PMCID: PMC12177334
- DOI: 10.1183/13993003.00198-2025
Comparability, acceptability and longitudinal adherence with digital emPHasis-10 in pulmonary arterial hypertension
Abstract
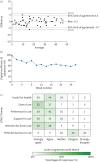
Digital emPHasis-10 is an app-based patient-reported outcome measure for trial or clinical use by patients with pulmonary hypertension. This letter demonstrates this novel tool's validity, longer-term adherence and high acceptability scores from patients.
Conflict of interest statement
Conflict of interest: D.G. Kiely and I. Armstrong were involved in the derivation of EmPHasis-10, and E.H. Davies is an employee of Aparito Ltd; these authors contributed to reviewing the manuscript, but statistical analysis was performed independently by J. Newman and F. Varian. J. Newman reports support for the present study from British Heart Foundation, and grants from Aparito Ltd and United Therapeutics. F. Varian has received educational funding from Janssen and is a Medical Research Council (UK) clinical fellow. D.G. Kiely reports grants from NIHR Biomedical Research Centre Sheffield, Ferrer, GSK and Janssen, and consulting fees from Acceleron, Altivant, Ferrer, Gossamer, Janssen, MSD and United Therapeutics. A.A.R. Thompson reports research funding from Heart Research UK, Janssen-Cilang Ltd and British Heart Foundation, and honoraria from Janssen-Cilad Ltd for lectures and education. A. Rothman reports support for the present study from Wellcome Trust Clinical Research Career Development Fellowship, EPSRC, Medical Research Council (UK) Experimental Medicine Award, BHF Clinical Research Training Fellowship and NIHR Biomedical Research Centre Sheffield, consulting fees from NXT Biomedical, Endotronix Inc., SoniVie, Neptune and Gradient, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Medtronic Inc., Abbott Laboratories, Endotronix Inc., Novartis, Janssen and Merck. M. Toshner reports support for the present study from NIHR Biomedical Research Centre Cambridge and NIHR HTA, and grants from GSK and Jansen. The remaining authors report no competing interests.
Figures
References
-
- Varian F, Burney R, Pearson C, et al. Selection of patient-reported outcome measures in pulmonary arterial hypertension clinical trials: a systematic review, meta-analysis and health-related quality of life framework. Eur Respir Rev 2025; 34: 250006. doi: 10.1183/16000617.0006-2025 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
