Particulate matter-related ITIH4 deficiency is associated with an emphysema phenotype of COPD through JNK-dependent and JNK-independent signalling
- PMID: 40404214
- PMCID: PMC12441582
- DOI: 10.1183/13993003.01610-2024
Particulate matter-related ITIH4 deficiency is associated with an emphysema phenotype of COPD through JNK-dependent and JNK-independent signalling
Abstract
Background: Prolonged exposure to airborne particulate matter (PM) is associated with emphysema and COPD; however, the precise underlying mechanism remains unclear. In a previous high-throughput screen, we identified inter-α-trypsin inhibitor heavy chain 4 (ITIH4) as a biomarker of long-term PM exposure. We hypothesised that ITIH4 is implicated in PM-related emphysema.
Methods: We investigated the association between ITIH4 expression and ambient PM exposure through a clinical cohort analysis (220 patients with COPD and 61 healthy participants) and in vitro studies.
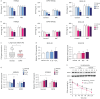
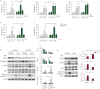
Results: The COPD cohort studies revealed significant correlations between emphysema severity, ambient PM exposure and serum ITIH4 levels. In primary small airway epithelial cells from COPD patients with low basal levels of ITIH4, exposure to PM and oxidative stress led to increased apoptosis. However, ITIH4 overexpression significantly inhibited oxidative-stress-induced apoptosis in normal and COPD airway epithelial cells. Acute exposure to hydrogen peroxide resulted in the rapid degradation of ITIH4 protein with no effect on transcriptional level, although ITIH4 gene expression is downregulated in the lung tissue of patients with COPD. A human apoptosis antibody array revealed that ITIH4 overexpression attenuated hydrogen-peroxide-induced apoptotic signalling. Furthermore, extracellular ITIH4 protein confers cytoprotective functions in cells exposed to PM or oxidative stress. Mechanistically, ITIH4 attenuated oxidative-stress-induced c-Jun N-terminal kinase (JNK) activation and β-catenin decrease. A deficiency of ITIH4 exacerbated the effects of oxidative stress.
Conclusions: We identified a novel pathogenetic mechanism involving ITIH4, where chronic exposure to air pollution induces or promotes emphysema.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures





Comment in
-
Pollution-induced ITIH4 deficiency: a gateway to novel targeted antioxidant therapy in COPD?Eur Respir J. 2025 Sep 17;66(3):2501097. doi: 10.1183/13993003.01097-2025. Print 2025 Sep. Eur Respir J. 2025. PMID: 40962424 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
