Impaired IgA mucosal immunity following lung transplantation: a potential trigger for bronchiolitis obliterans syndrome
- PMID: 40404215
- PMCID: PMC12591150
- DOI: 10.1183/13993003.02212-2024
Impaired IgA mucosal immunity following lung transplantation: a potential trigger for bronchiolitis obliterans syndrome
Abstract
Rationale: Bronchiolitis obliterans syndrome (BOS) limits long-term survival after lung transplantation (LuTx) and may be triggered by infections. As immunoglobulin (Ig)A is crucial to ensure adequate mucosal immunity, we explored whether IgA-related mucosal immunity is impaired in BOS.
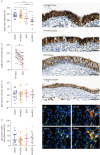
Methods: 60 LuTx recipients from the Cohort for Lung Transplantation (COLT) cohort were included retrospectively. All participants were in stable condition within the first year post-transplant. At 3.5 years post-LuTx, 30 remained stable and 30 had developed BOS. Bronchoalveolar lavage fluid (BALF) and sera collected pre-transplant and at 6 (M6) and 12 months (M12) post-transplant were assessed for monomeric IgA, secretory (S)-IgA, secretory component and cytokine profiling. Second, bronchiolar polymeric Ig receptor (pIgR) expression and subepithelial IgA-producing B-cell numbers were compared across graft tissue samples from 54 LuTx recipients classified as stable, pre-BOS, BOS or end-stage BOS.
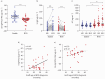
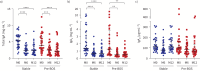
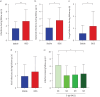
Results: S-IgA levels in BALF decreased between M6 and M12 (p=0.0001) and were reduced in BOS patients at M12 (p=0.0018). Patients with lower S-IgA levels had higher infection rates. BOS patients exhibited elevated secretory component levels in serum (p<0.01). Both reduced S-IgA in BALF and increased secretory component in serum were associated with higher risk of BOS. Lastly, a reduction in bronchiolar pIgR expression was observed in BOS patients (p=0.0001), that paralleled BOS severity.
Conclusions: This study demonstrates an early impairment of mucosal IgA immunity in LuTx patients, which was linked to the later development of BOS, suggesting that IgA-related markers may serve as early predictors of BOS onset.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures


Comment in
-
Beyond systemic immunity: the mucosal frontier in bronchiolitis obliterans syndrome pathogenesis.Eur Respir J. 2025 Nov 6;66(5):2501129. doi: 10.1183/13993003.01129-2025. Print 2025 Nov. Eur Respir J. 2025. PMID: 41198404 No abstract available.
References
-
- Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult lung transplantation report – 2021; focus on recipient characteristics. J Heart Lung Transplant 2021; 40: 1060–1072. doi: 10.1016/j.healun.2021.07.021 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
