Effect of acupoint hot compress on relieving pain in primiparous women during the latent phase of the first stage of labour: a study protocol for a prospective, multicentre, randomised controlled clinical trial
- PMID: 40404328
- PMCID: PMC12096971
- DOI: 10.1136/bmjopen-2024-094226
Effect of acupoint hot compress on relieving pain in primiparous women during the latent phase of the first stage of labour: a study protocol for a prospective, multicentre, randomised controlled clinical trial
Abstract
Introduction: Labour pain is an unavoidable feature of childbirth and is characterised by extreme intensity. Adequate pain management is thus essential not only from the aspect of physiological pain but also due to the adverse effects of pain on the psychological well-being of parturients. Many studies have shown the benefits of acupoint hot compress. However, to date, little is known about its use for alleviating labour pain. The purpose is to evaluate the effect of acupoint hot compress on relieving pain in primiparous women during the latent phase of the first stage of labour, as well as its effects on key maternal and neonatal outcomes.
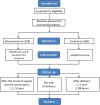
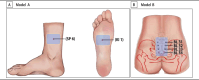
Methods and analysis: This prospective, multicentre, randomised controlled trial will be conducted across 18 institutions in China from January 2024 to August 2025. A total of 1100 primiparous women aged 20-34 years, with singleton pregnancies at 37-41 weeks of gestation, will be enrolled and randomly allocated to two groups using a central stratified block randomisation method. The controls will be treated only with obstetrical care, while those in the intervention group will receive the same obstetrical care as the control group, with the addition of acupoint hot compress therapy at 42±2°C for 4 hours, starting 1 hour after the onset of regular uterine contractions during the latent phase of labour. The primary outcome will be the pain intensity measured at 1, 3 and 5 hours after the onset of regular uterine contractions using a Visual Analog Scale.
Ethics and dissemination: The study has been approved by the ethics committee of Women's Hospital, School of Medicine, Zhejiang University (No. IRB-20230379-R). The results of the main trial will be submitted for publication in a peer-reviewed journal.
Trial registration number: This trial is registered at Chinese Clinical Trial Registry, ChiCTR2300079244.
Keywords: COMPLEMENTARY MEDICINE; PAIN MANAGEMENT; Pregnant Women; Puerperal Disorders.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical